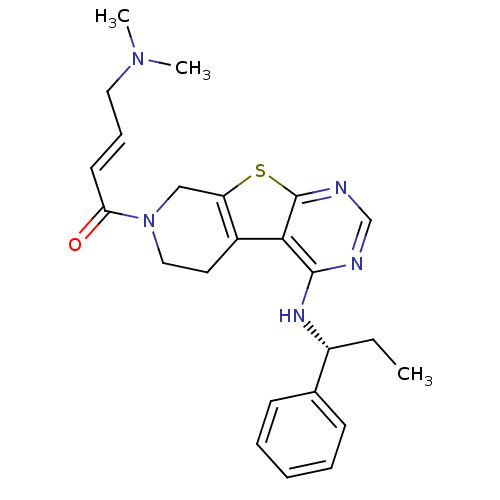
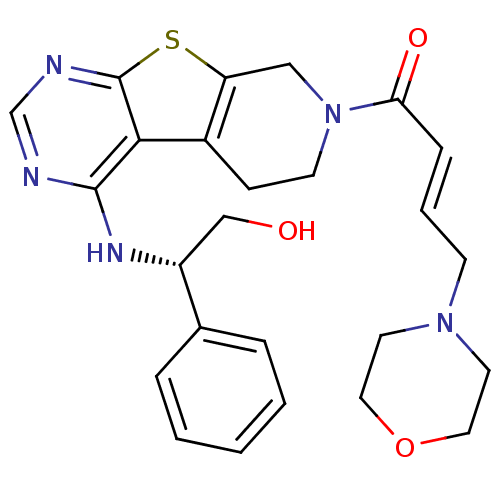
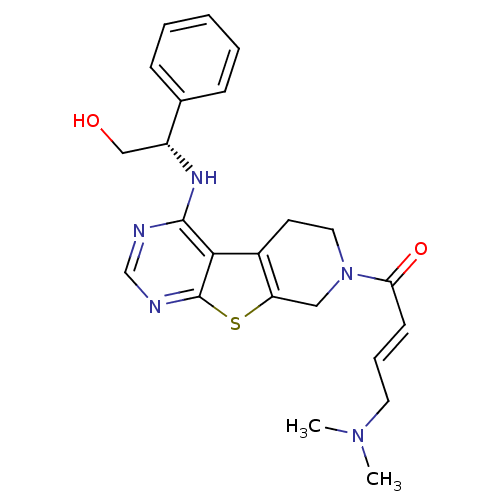
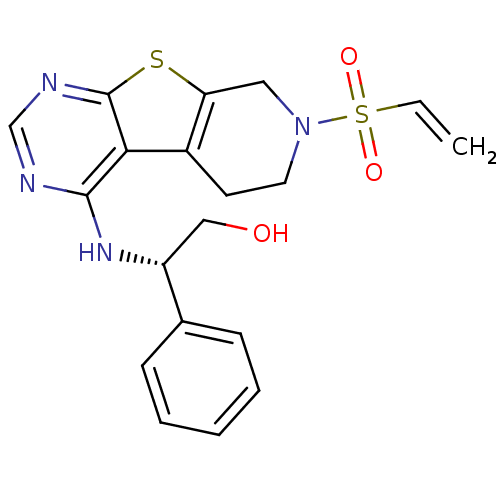
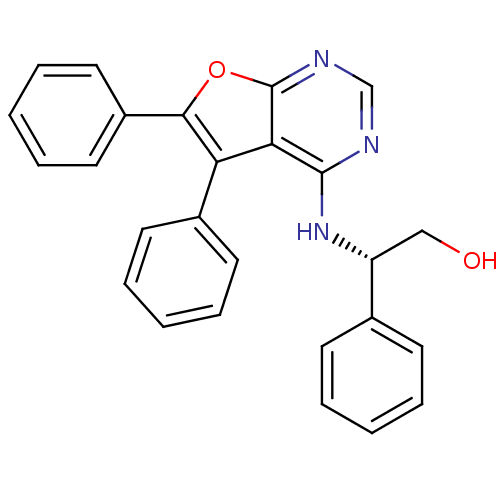
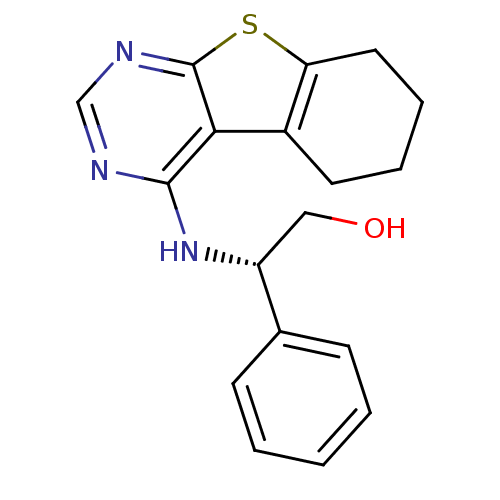
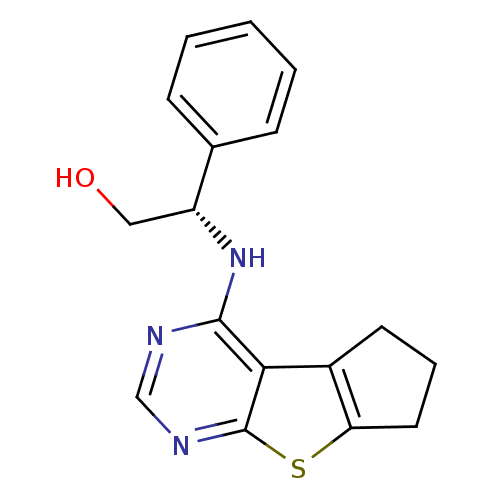
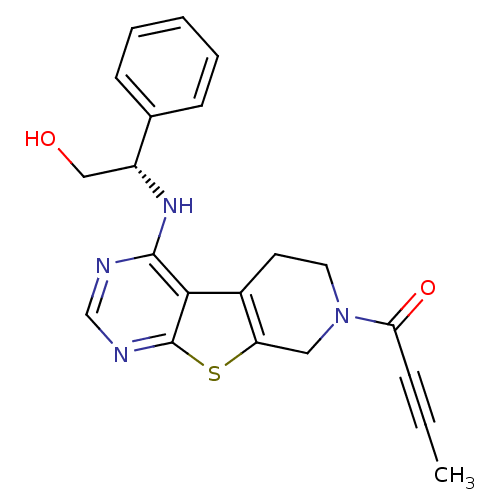
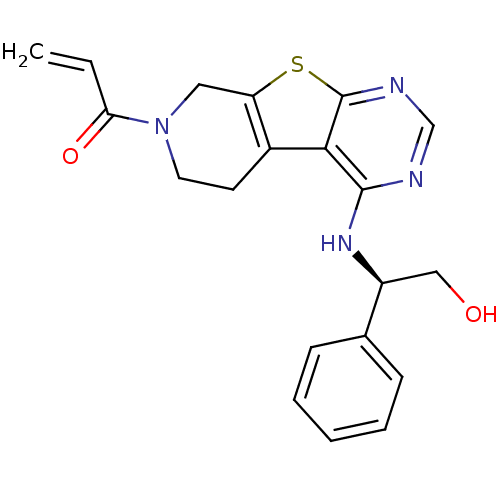
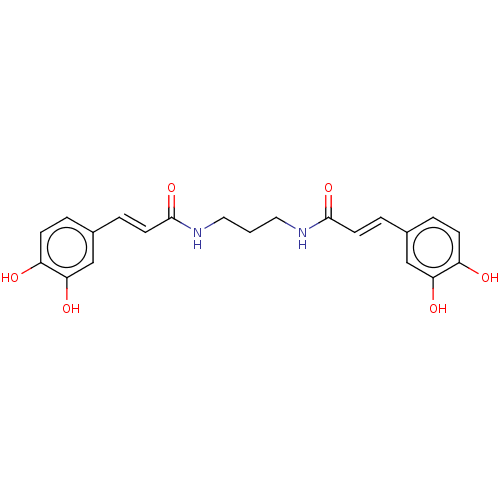
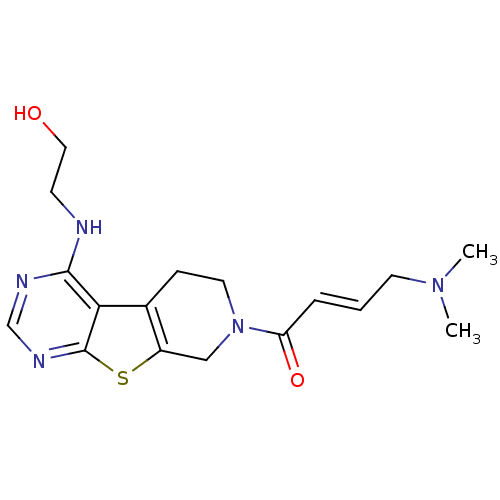
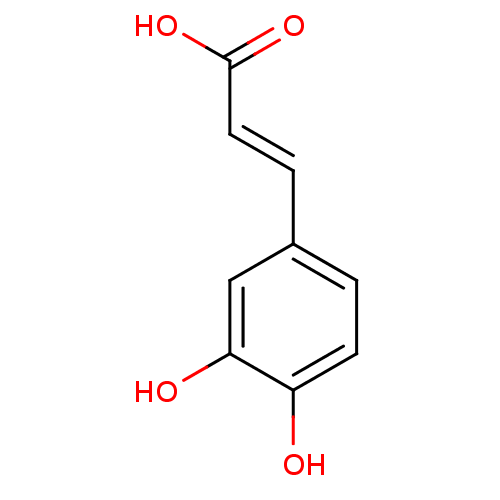
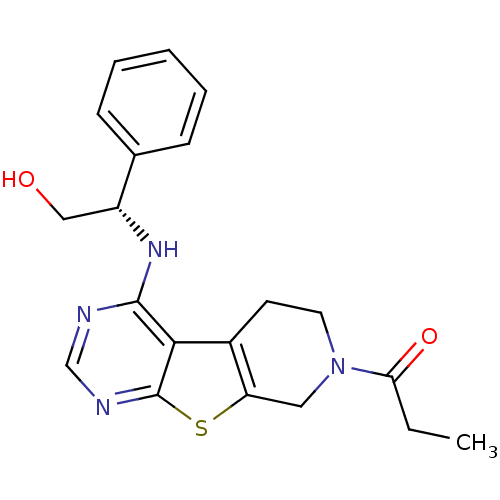
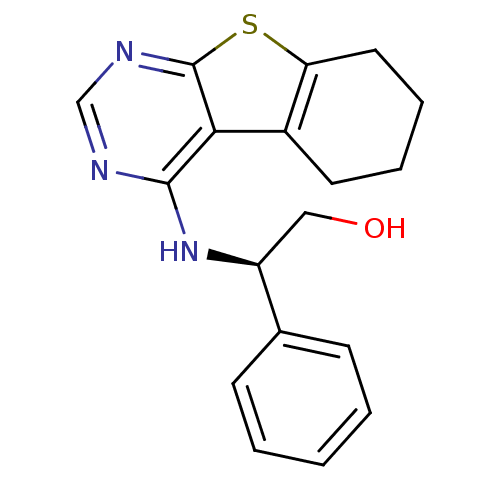
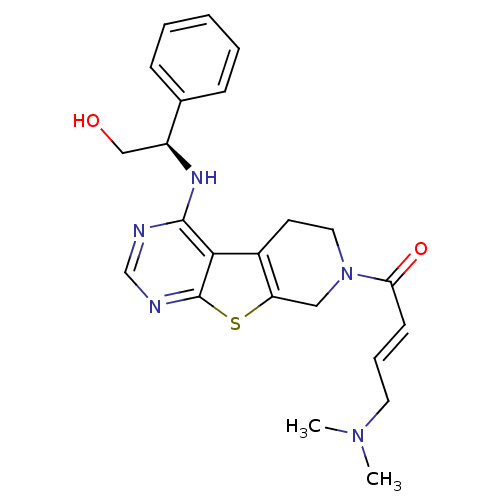
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Genomics Research Center
Curated by ChEMBL
Genomics Research Center
Curated by ChEMBL
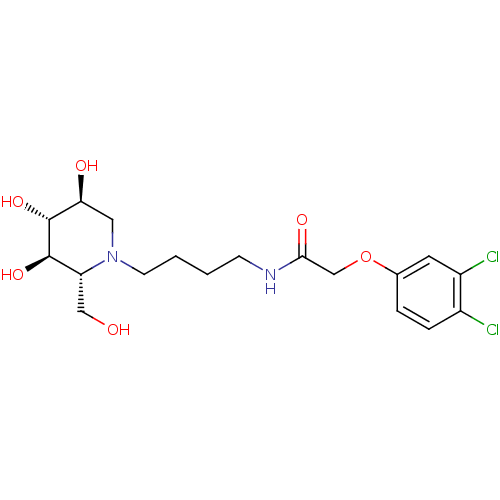
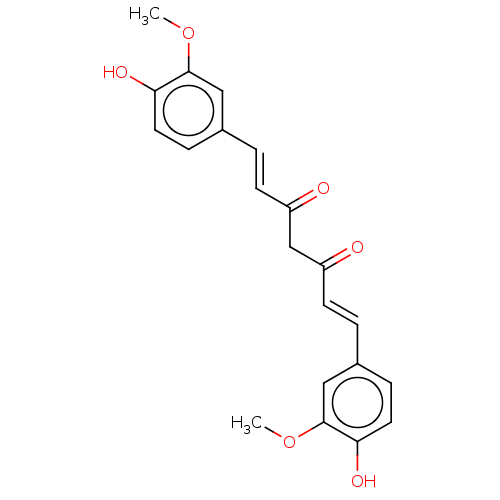
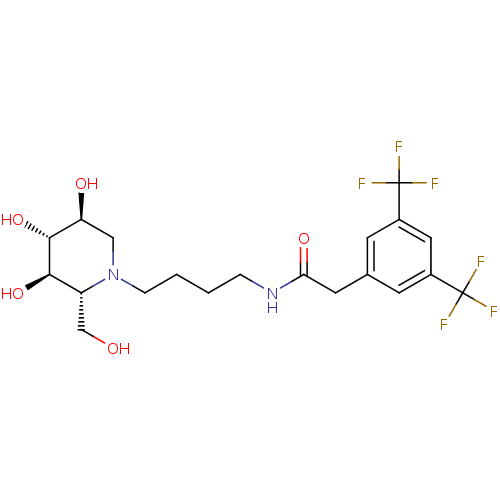
Affinity DataKi: 71nMAssay Description:Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot methodMore data for this Ligand-Target Pair
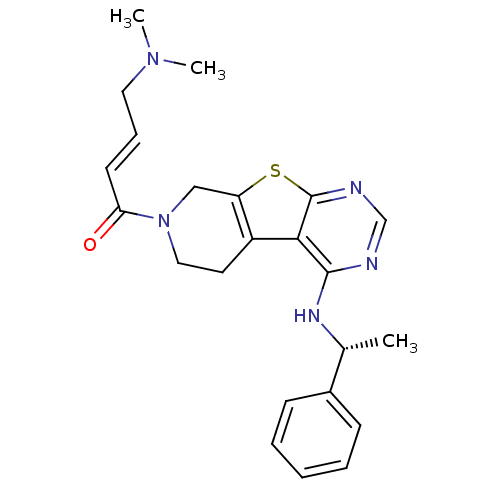
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Genomics Research Center
Curated by ChEMBL
Genomics Research Center
Curated by ChEMBL
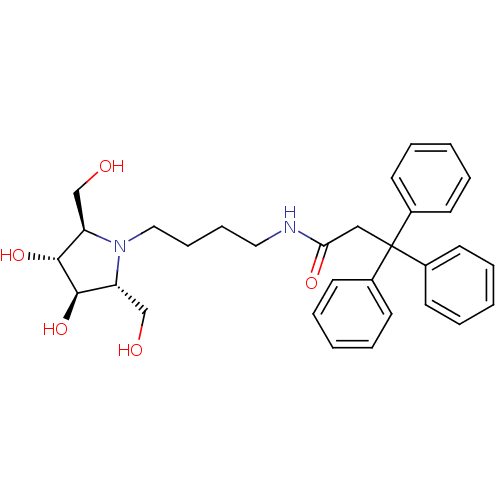
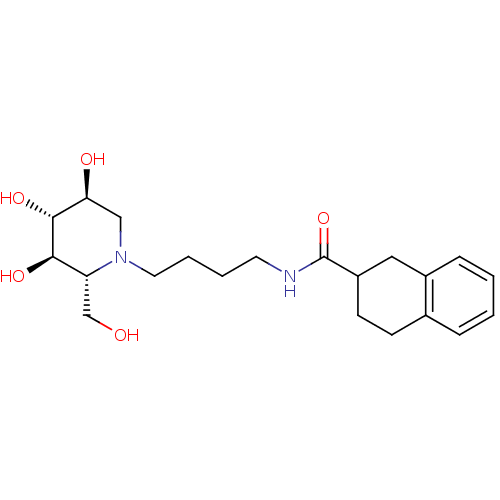
Affinity DataKi: 400nMAssay Description:Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot methodMore data for this Ligand-Target Pair
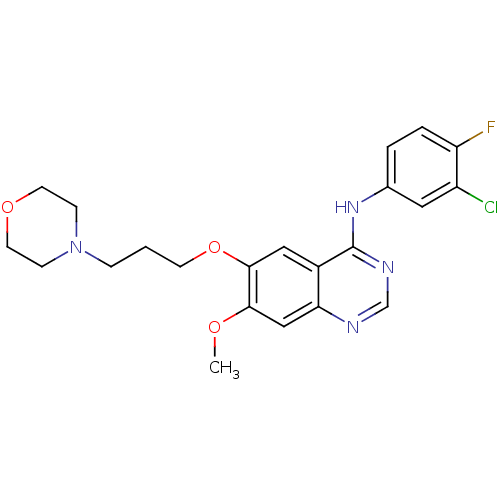
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Genomics Research Center
Curated by ChEMBL
Genomics Research Center
Curated by ChEMBL
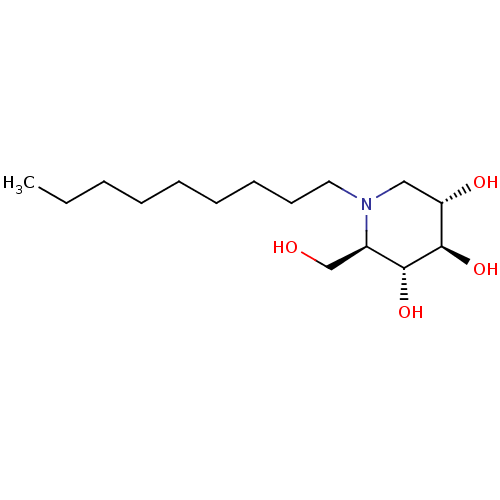
Affinity DataKi: 400nMAssay Description:Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot methodMore data for this Ligand-Target Pair
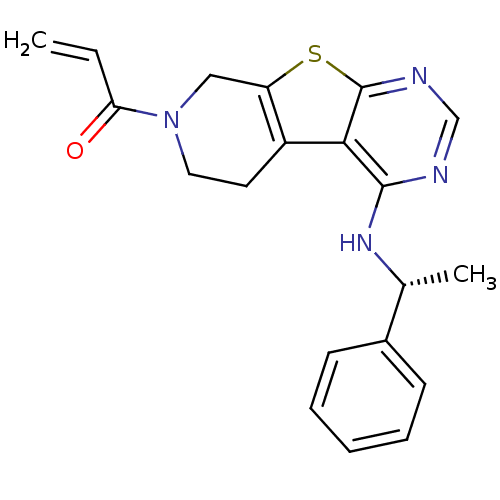
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Genomics Research Center
Curated by ChEMBL
Genomics Research Center
Curated by ChEMBL
Affinity DataKi: 1.00E+3nMAssay Description:Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot methodMore data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Genomics Research Center
Curated by ChEMBL
Genomics Research Center
Curated by ChEMBL
Affinity DataKi: 1.10E+3nMAssay Description:Non-competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot methodMore data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Genomics Research Center
Curated by ChEMBL
Genomics Research Center
Curated by ChEMBL
Affinity DataKi: 1.30E+3nMAssay Description:Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot methodMore data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Genomics Research Center
Curated by ChEMBL
Genomics Research Center
Curated by ChEMBL
Affinity DataKi: 3.20E+3nMAssay Description:Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot methodMore data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Genomics Research Center
Curated by ChEMBL
Genomics Research Center
Curated by ChEMBL
Affinity DataKi: 4.10E+3nMAssay Description:Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot methodMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
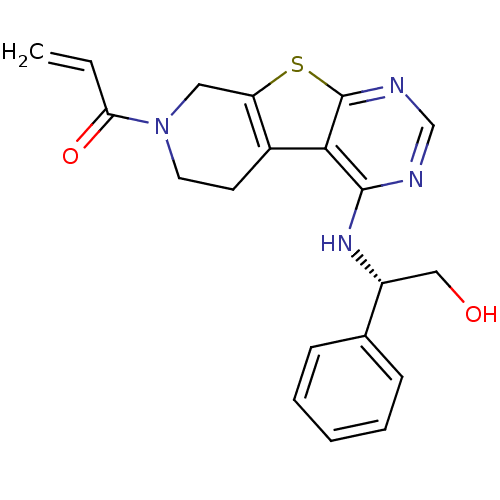
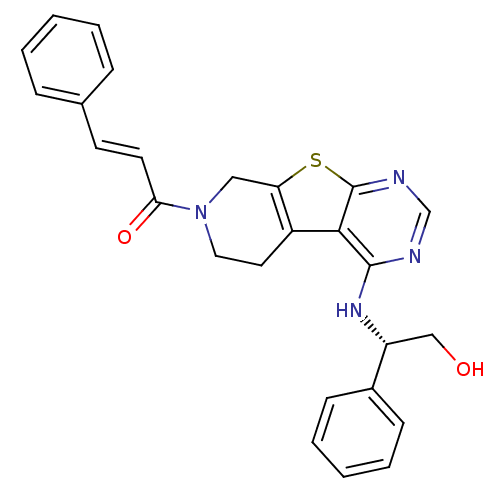
Affinity DataIC50: 1.09E+3nMAssay Description:Inhibition of EGFRMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
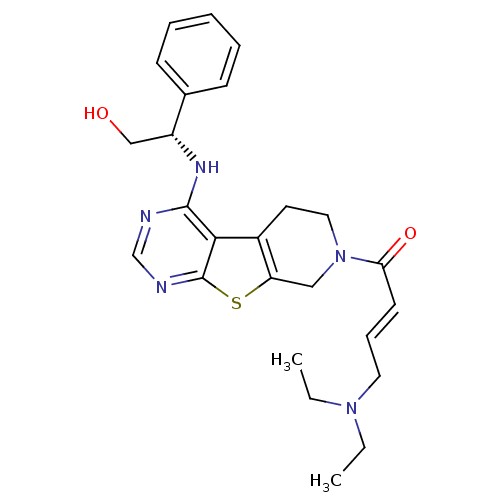
Affinity DataIC50: 1.27E+3nMAssay Description:Inhibition of EGFRMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 3.06E+3nMAssay Description:Inhibition of EGFRMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: >3.24E+3nMAssay Description:Inhibition of EGFRMore data for this Ligand-Target Pair
Affinity DataIC50: 3.40E+3nMAssay Description:Inhibition of amyloid beta (1 to 42) fibrillization (unknown origin) incubated with agitation for 1 min every hr measured over 80 hrs by thioflavin-T...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 3.50E+3nMAssay Description:Inhibition of EGFRMore data for this Ligand-Target Pair
Affinity DataIC50: 4.20E+3nMAssay Description:Disaggregation of amyloid beta (1 to 42 residues) (unknown origin) preformed fibrils incubated with agitation for 1 min every hr measured over 80 hrs...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 4.88E+3nMAssay Description:Inhibition of EGFRMore data for this Ligand-Target Pair
Affinity DataIC50: 4.90E+3nMAssay Description:Inhibition of amyloid beta (1 to 42) fibrillization (unknown origin) incubated with agitation for 1 min every hr measured over 80 hrs by thioflavin-T...More data for this Ligand-Target Pair
Affinity DataIC50: 1.56E+4nMAssay Description:Inhibition of amyloid beta (1 to 42) fibrillization (unknown origin) incubated with agitation for 1 min every hr measured over 80 hrs by thioflavin-T...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of EGFR at 20 uMMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of EGFR at 20 uMMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of EGFR at 20 uMMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of EGFR at 20 uMMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of EGFR at 20 uMMore data for this Ligand-Target Pair




 3D Structure (crystal)
3D Structure (crystal)