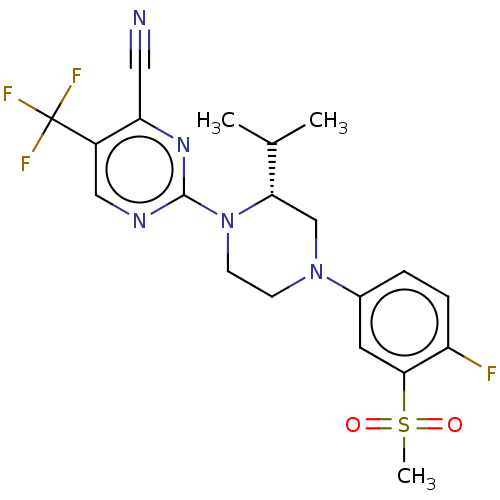
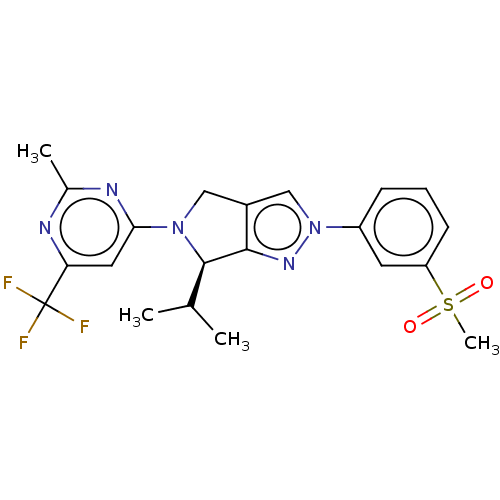
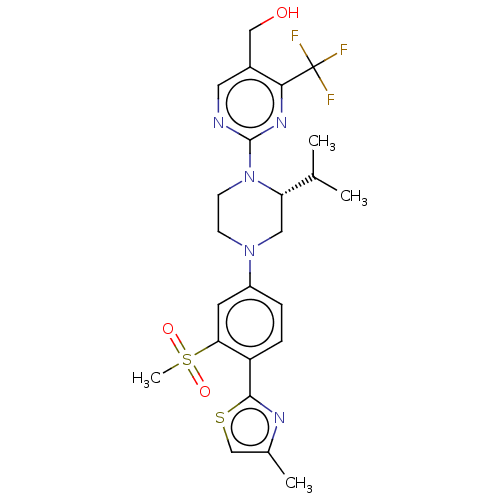
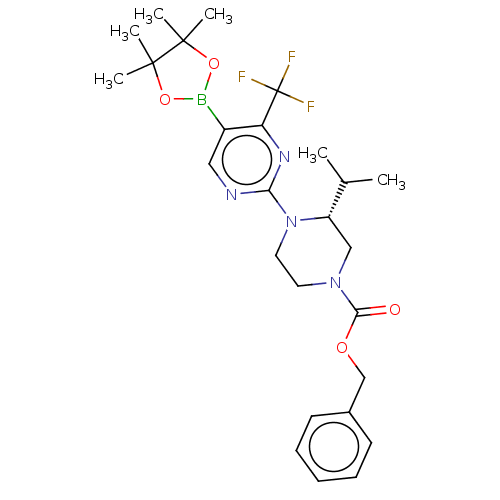
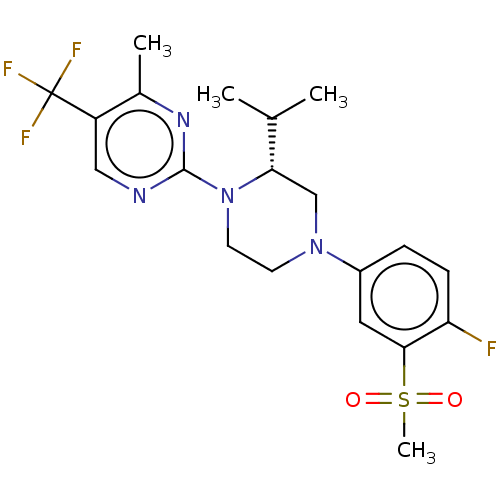
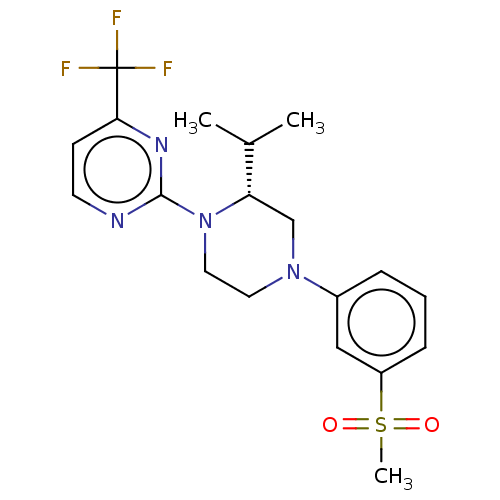
TargetVoltage-dependent L-type calcium channel subunit alpha-1C(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 0.25nMAssay Description:Inhibition of [3H]nitrendipine binding to L-type calcium channel dihydropyridine site of porcine cardiac sarcolemma membrane vesiclesMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2B(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
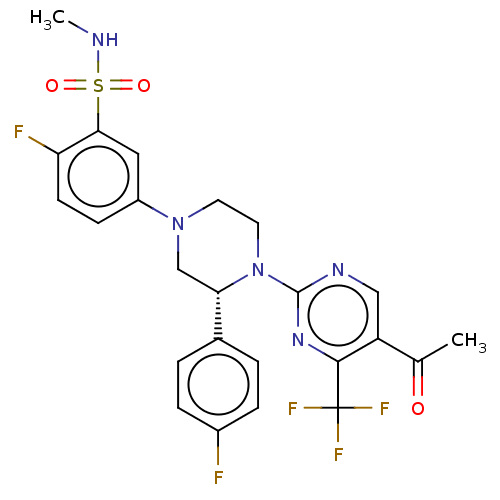
Affinity DataKi: 0.400nMAssay Description:Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 0.600nMAssay Description:Displacement of NMDA receptor-specific [3H]-ifenprodil binding to recombinant human NMDA receptor, NR2B subtype expressed in L cellsMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2B(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 0.680nMAssay Description:Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Displacement of NMDA receptor-specific [3H]-ifenprodil binding to recombinant human NMDA receptor, NR2B subtype expressed in L cellsMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2B(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 0.820nMAssay Description:Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2B(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 0.850nMAssay Description:Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2B(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 0.990nMAssay Description:Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel...More data for this Ligand-Target Pair
TargetVoltage-dependent L-type calcium channel subunit alpha-1C(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Inhibition of [3H]nitrendipine binding to L-type calcium channel dihydropyridine site of porcine cardiac sarcolemma membrane vesiclesMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
TargetVoltage-dependent L-type calcium channel subunit alpha-1C(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Inhibition of [3H]nitrendipine binding to L-type calcium channel dihydropyridine site of porcine cardiac sarcolemma membrane vesiclesMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin)More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2B(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.20nMAssay Description:Displacement of NMDA receptor-specific [3H]-ifenprodil binding to recombinant human NMDA receptor, NR2B subtype expressed in L cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2B(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.5nMAssay Description:Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Binding affinity to NMDA NR2B receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 2.20nMAssay Description:Binding affinity to NMDA NR2B receptorMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2B(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2B(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.20nMAssay Description:Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.40nMAssay Description:Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cellsMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.60nMAssay Description:Binding affinity to NMDA NR2B receptorMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.70nMAssay Description:Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cellsMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.90nMAssay Description:Displacement of NMDA receptor-specific [3H]-ifenprodil binding to recombinant human NMDA receptor, NR2B subtype expressed in L cellsMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair

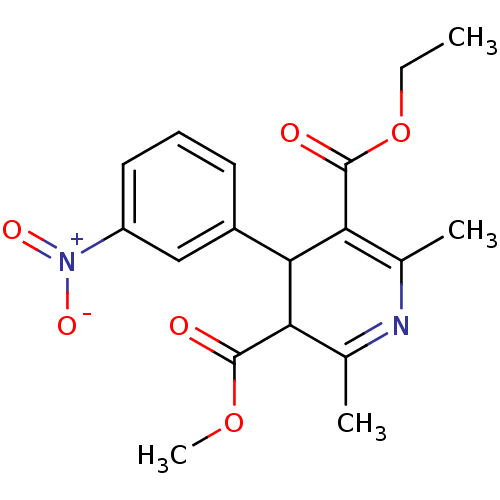
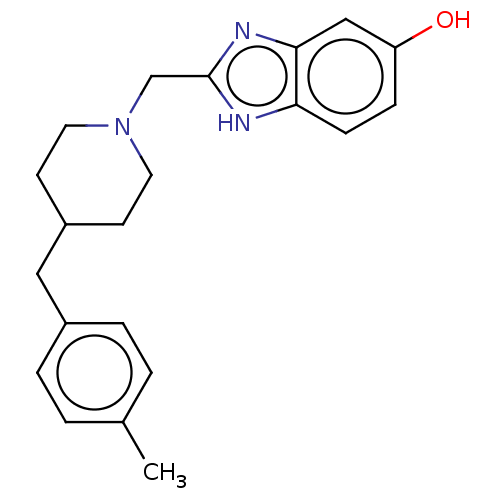
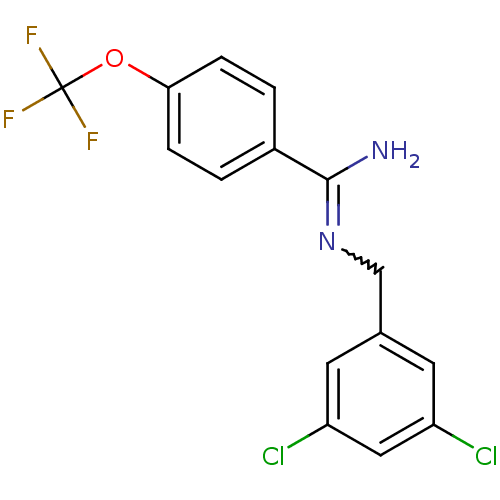
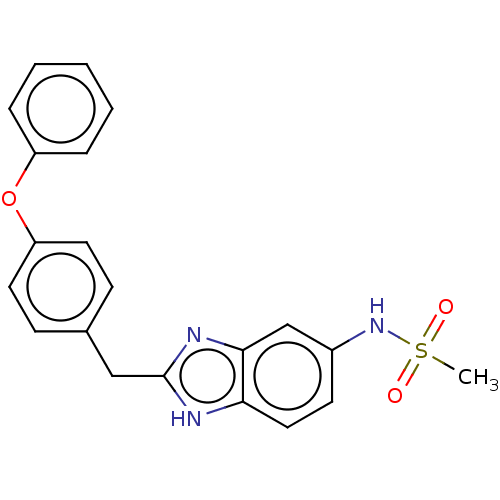
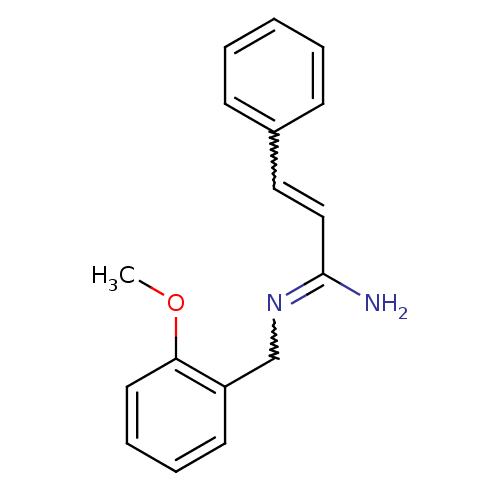
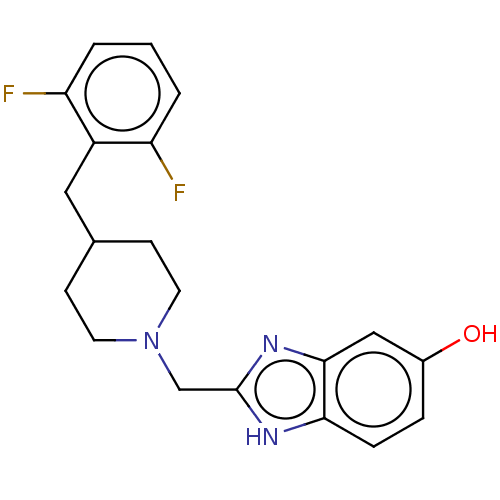
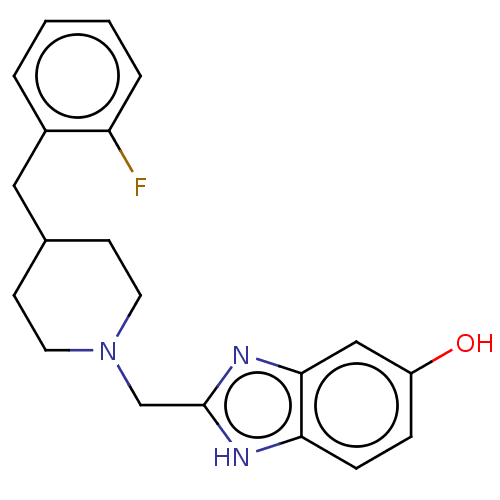

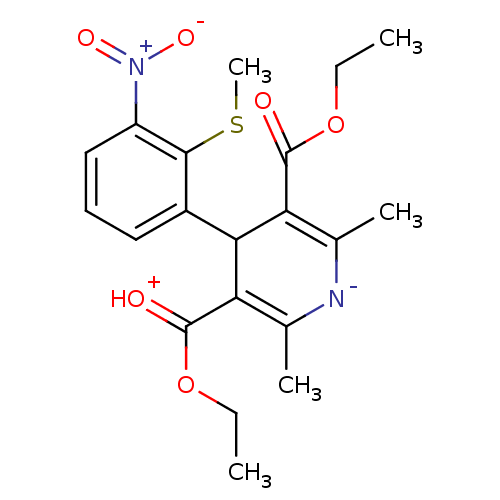
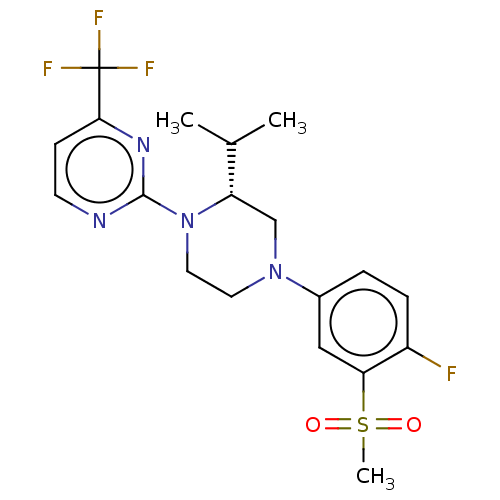
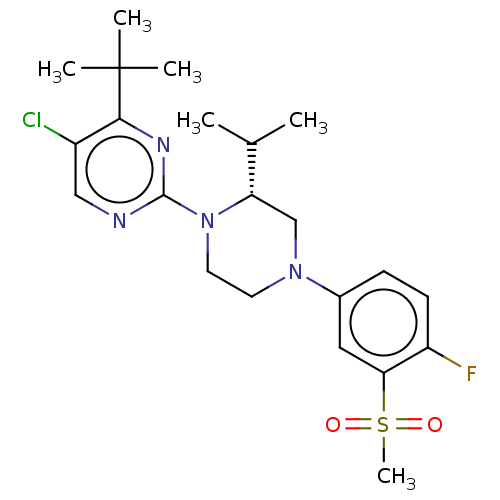
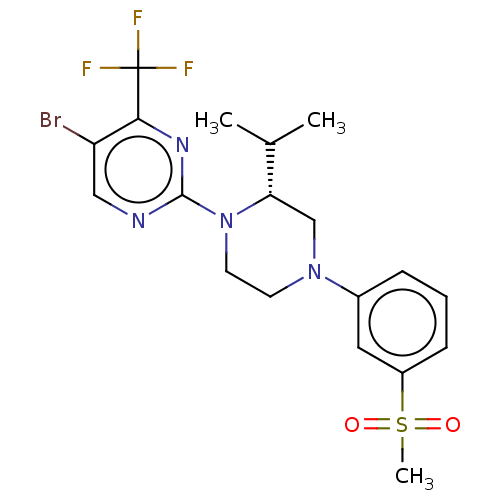
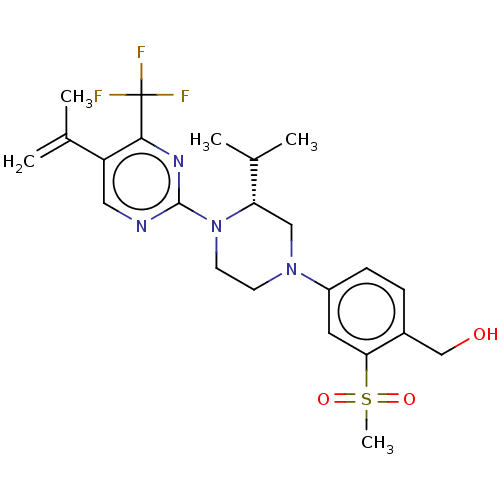

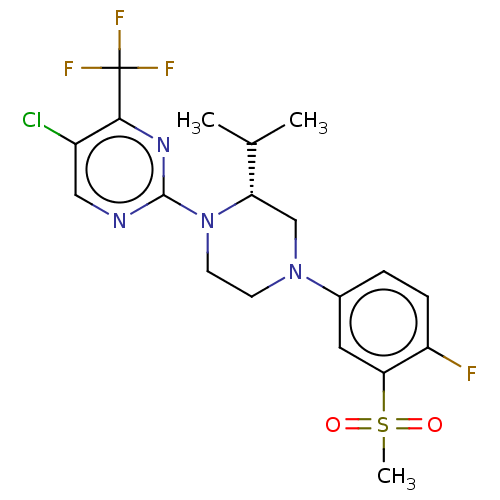
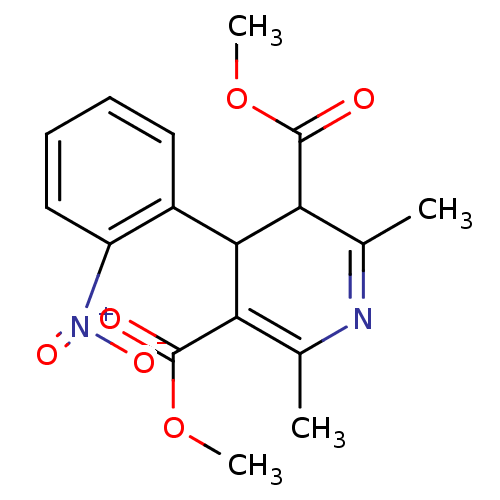
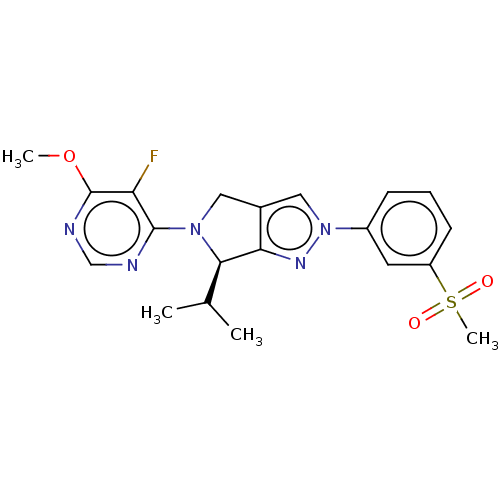
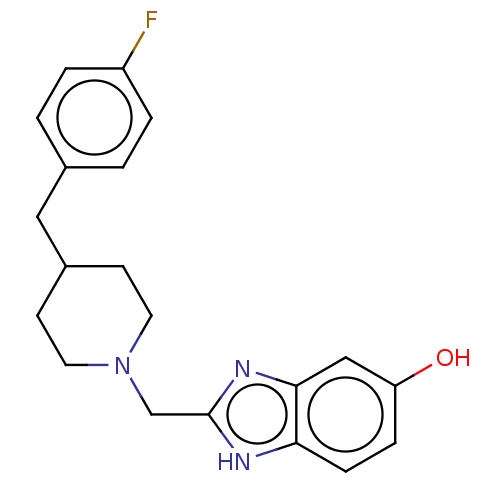

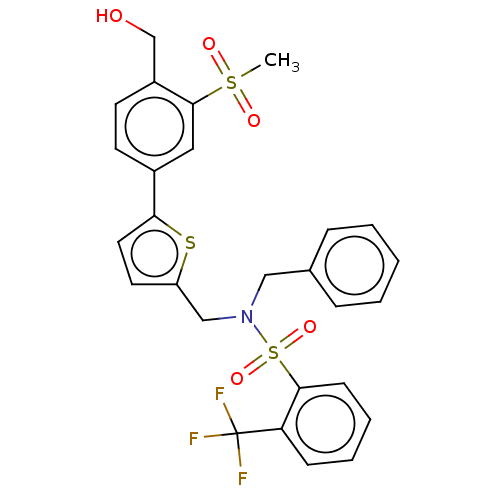
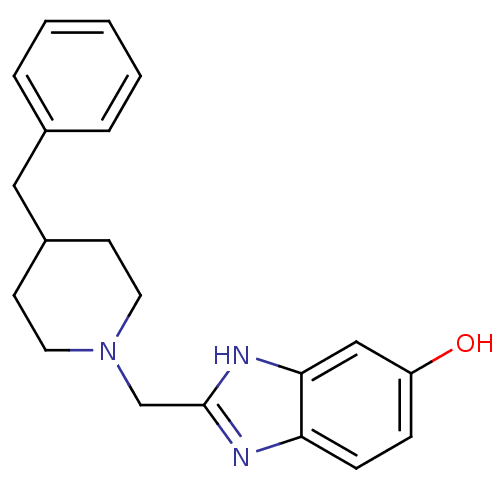
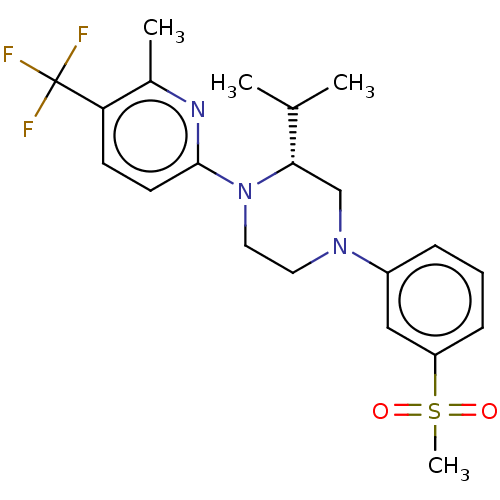
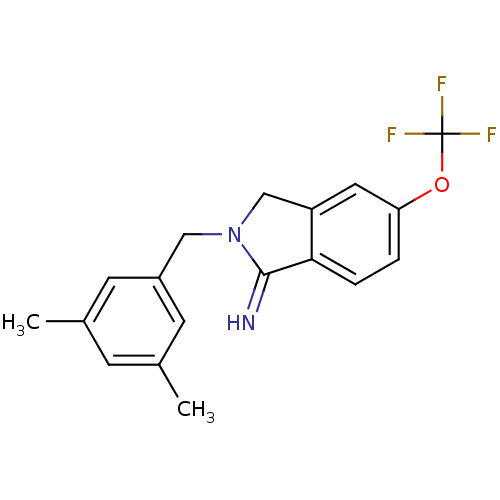
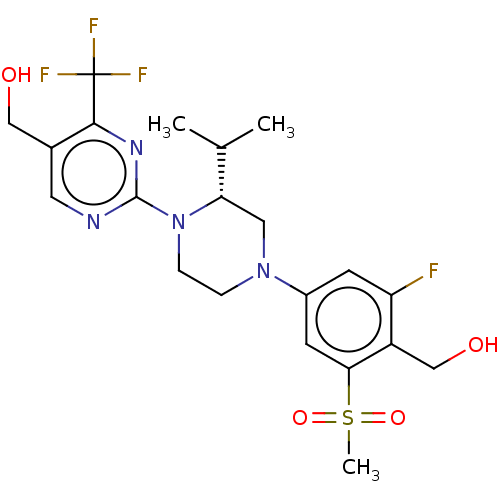
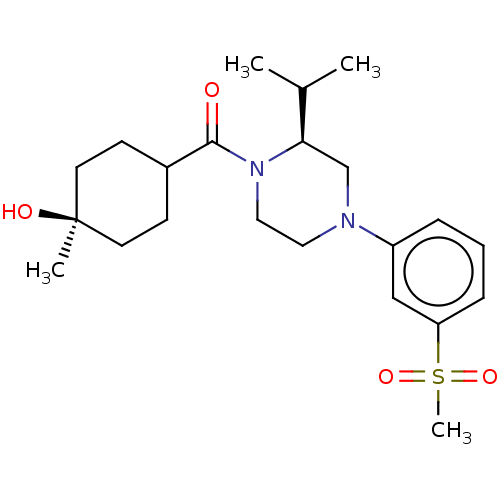
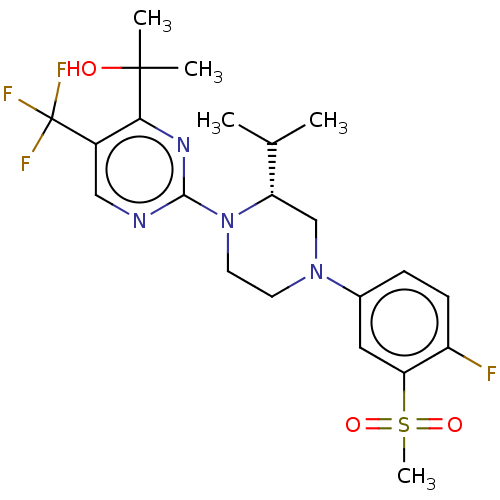
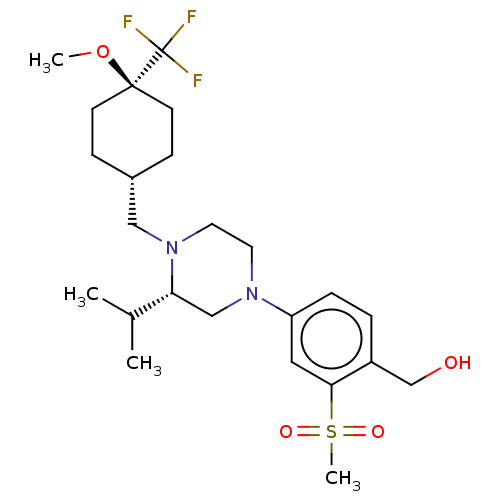
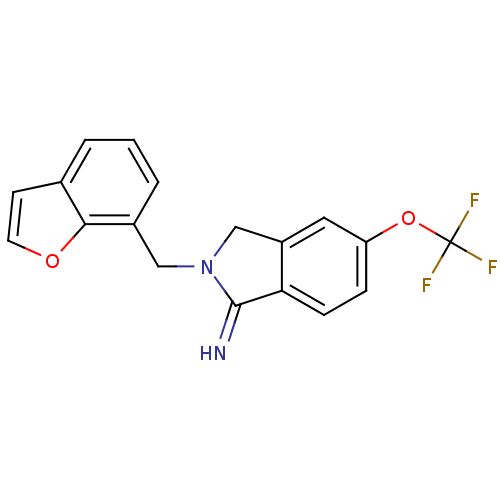
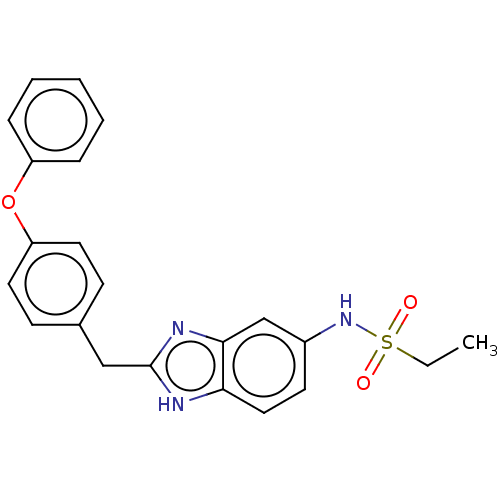
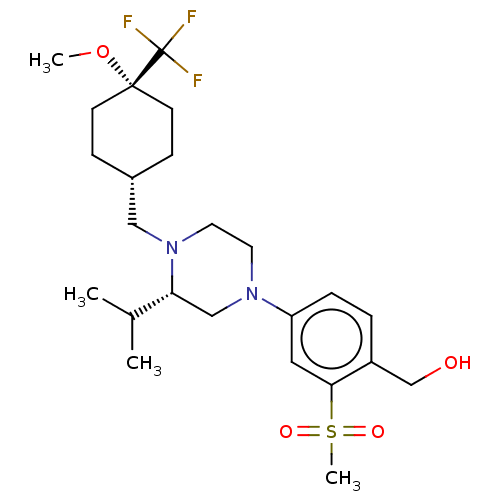
 3D Structure (crystal)
3D Structure (crystal)