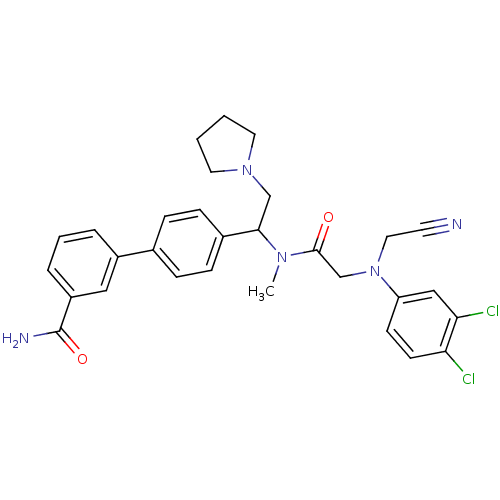
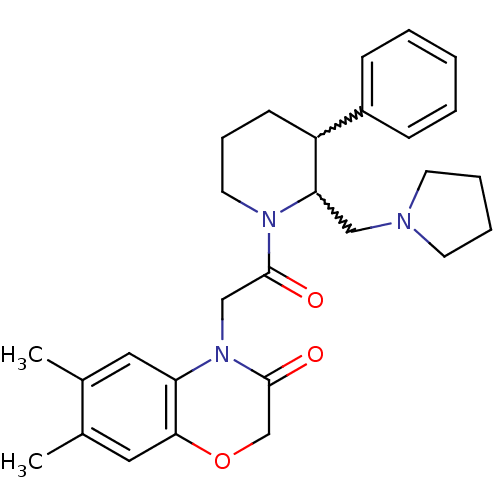
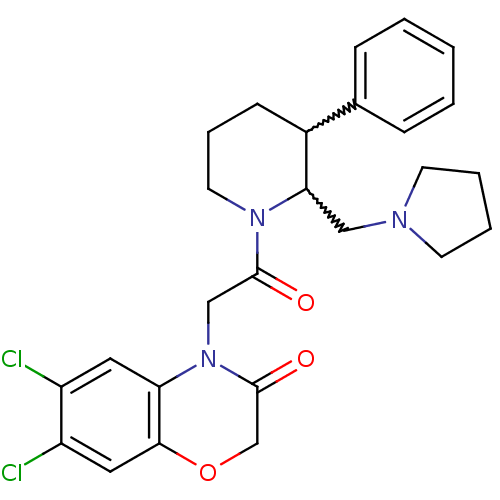
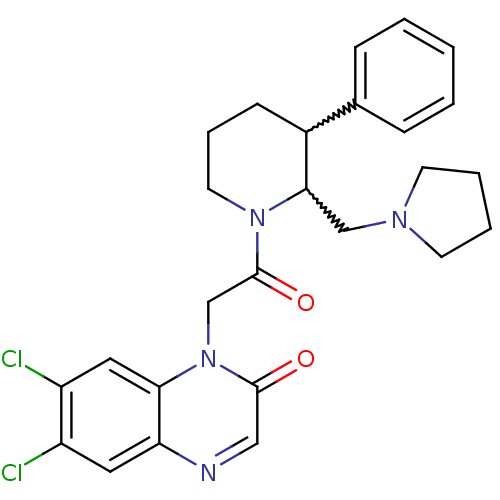
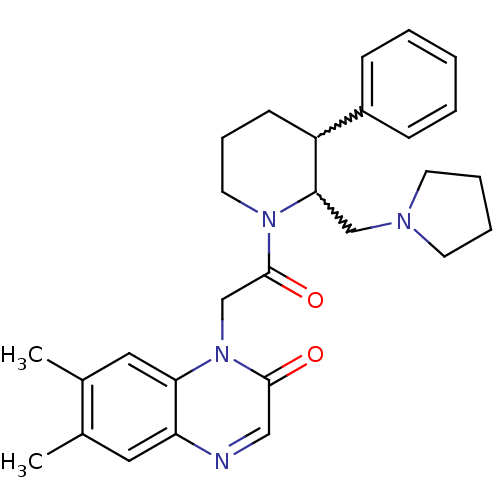
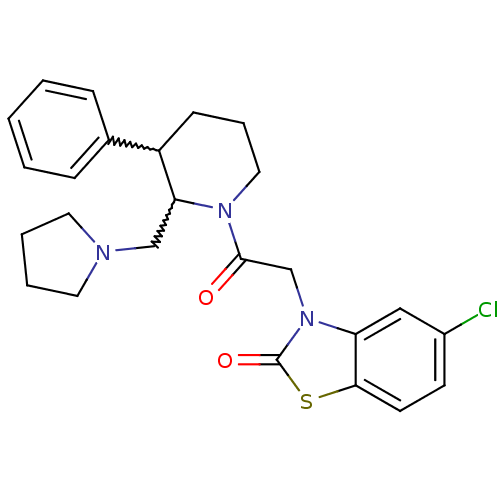
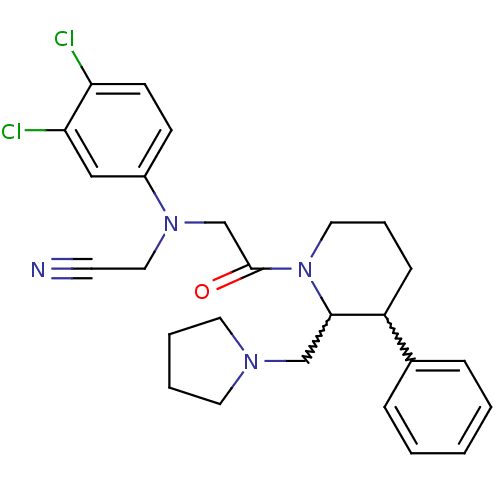
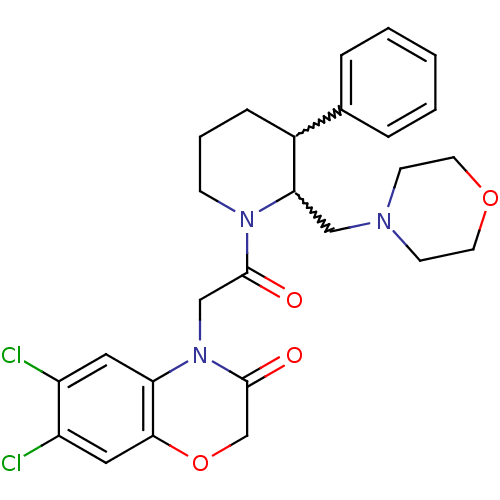
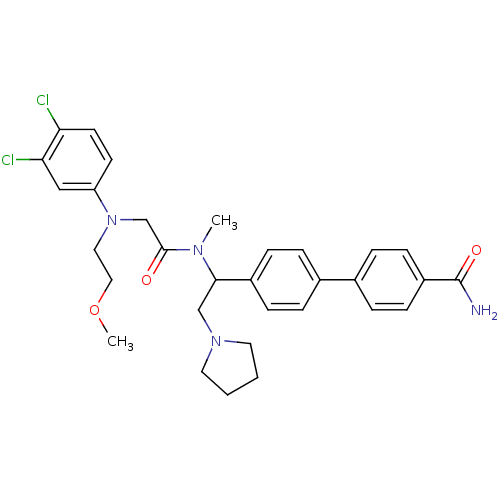
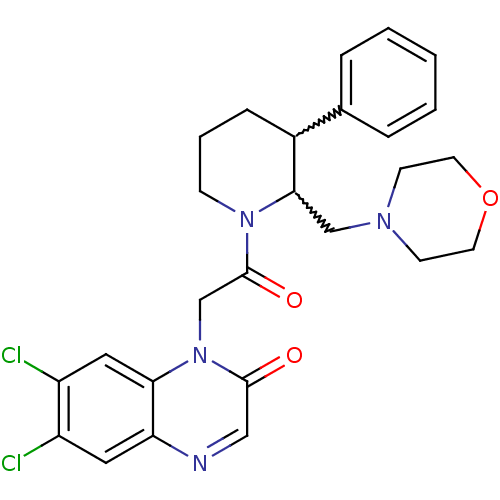
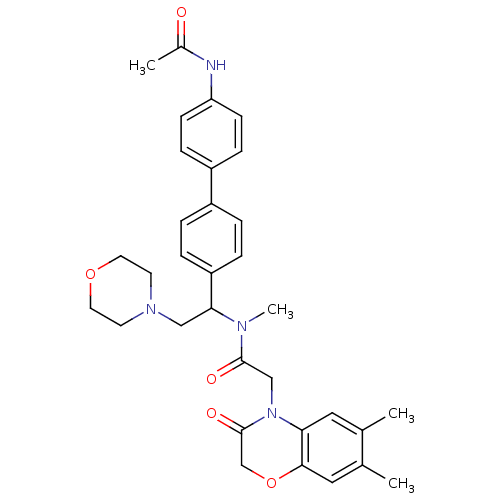
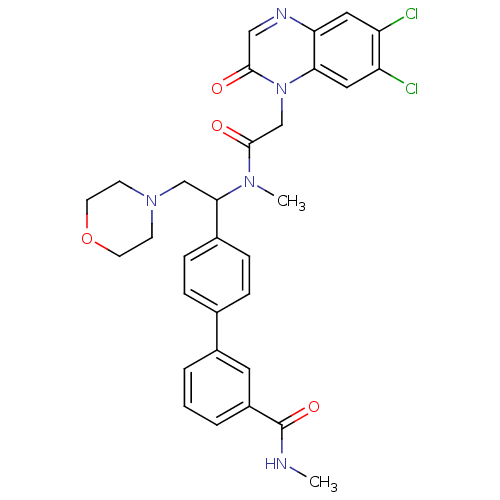
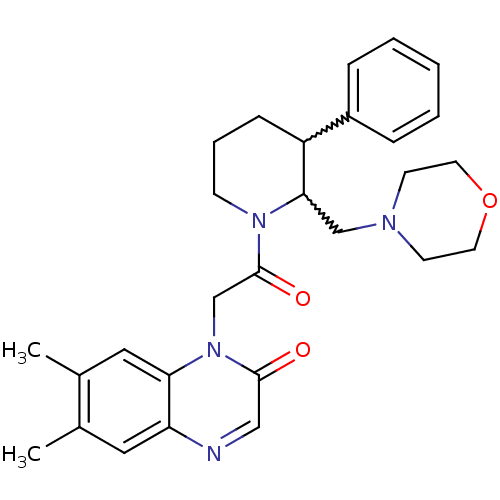
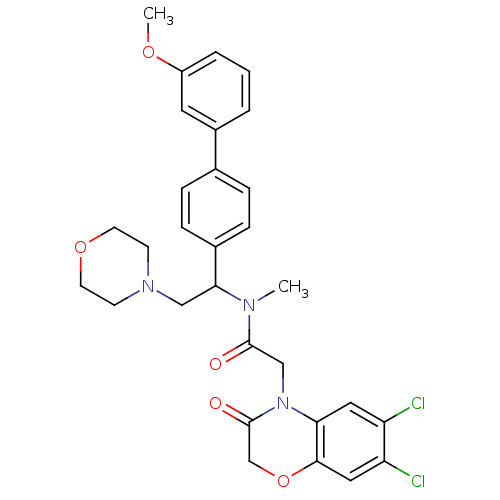
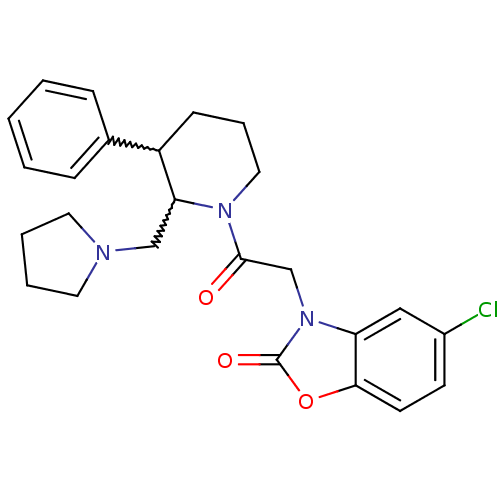
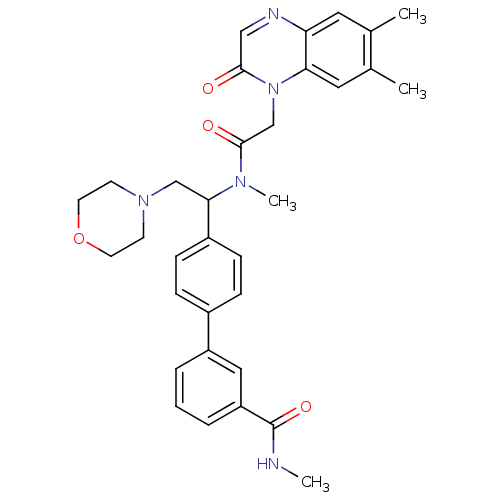
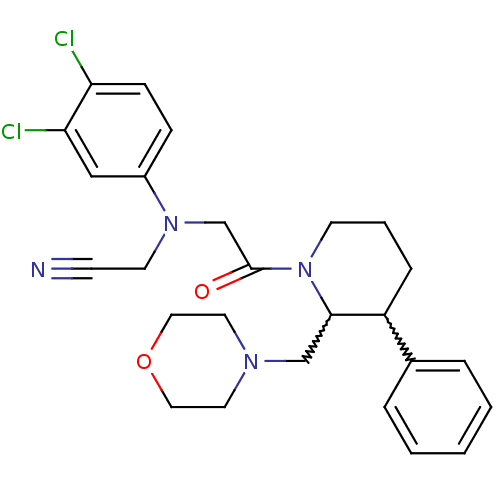
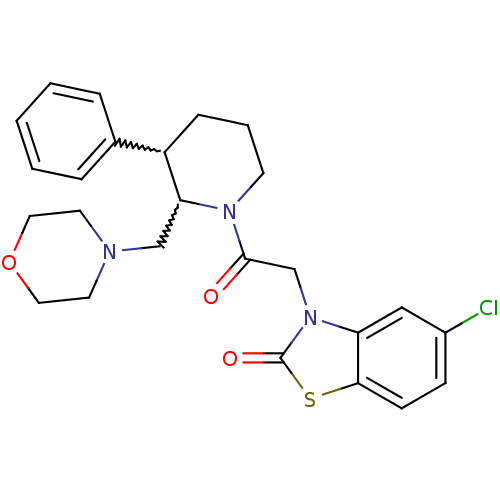
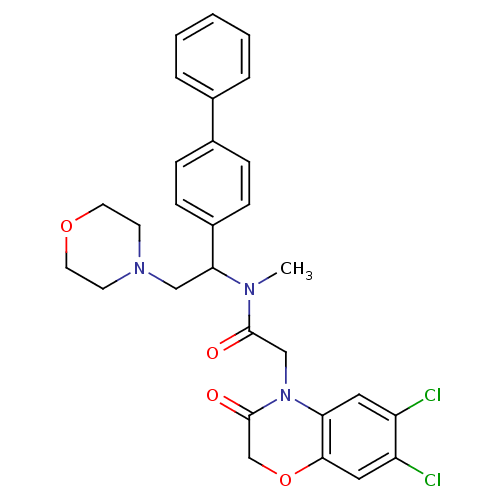
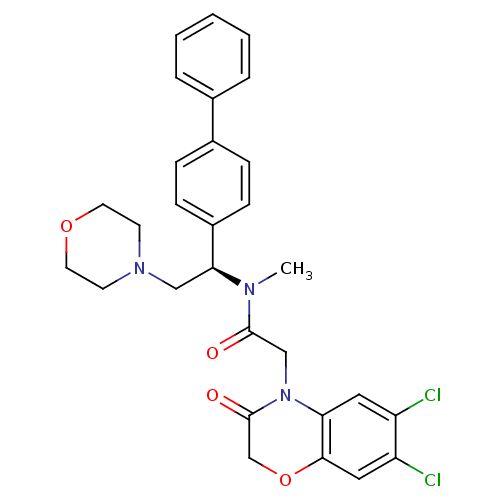
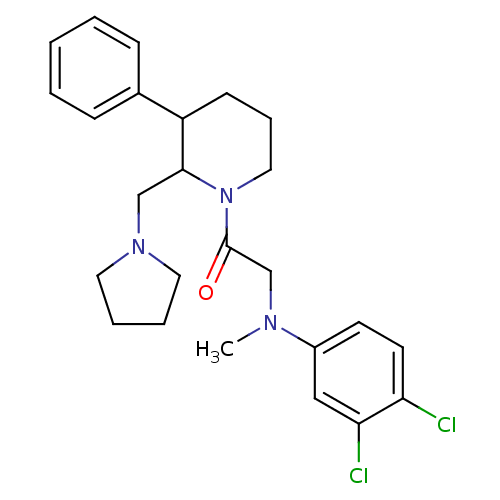
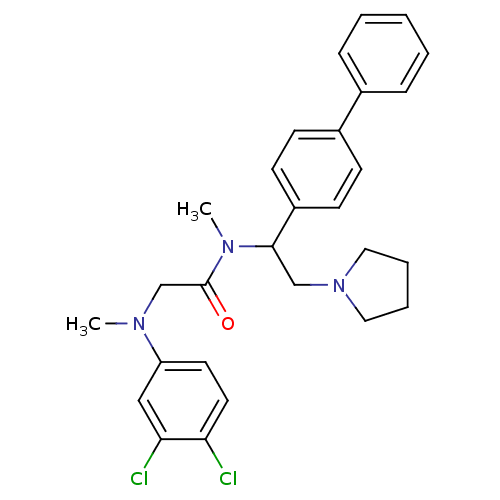
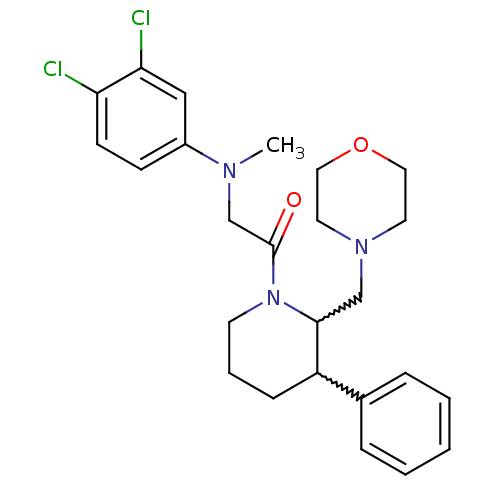
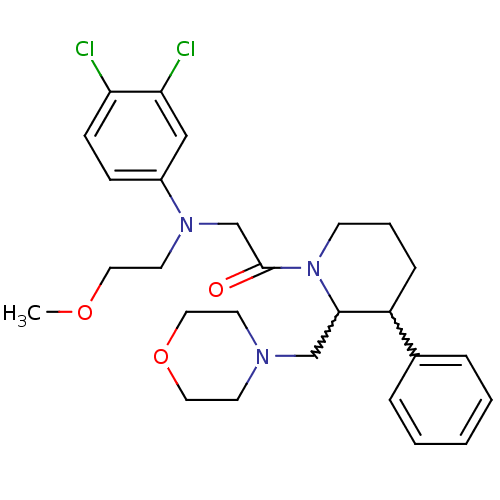
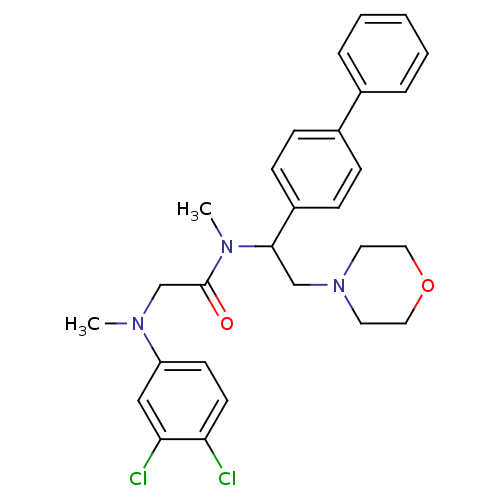
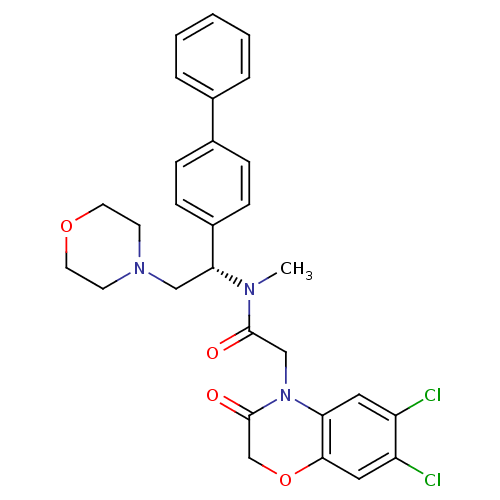
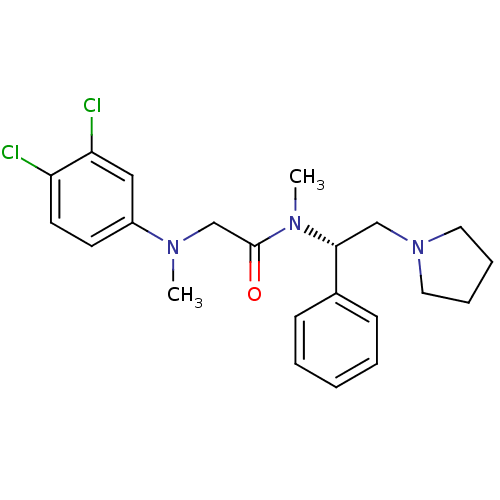
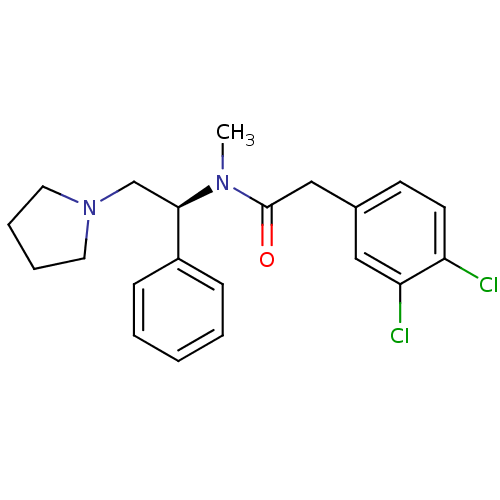
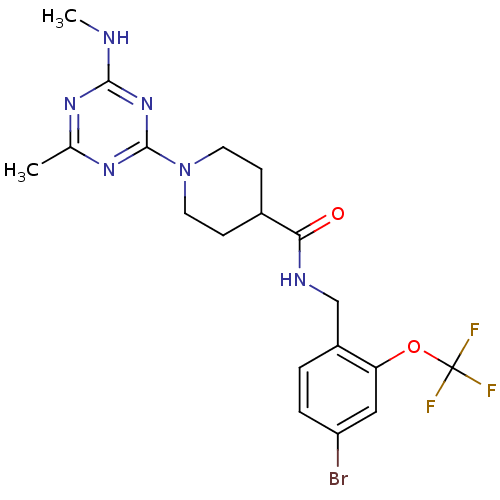
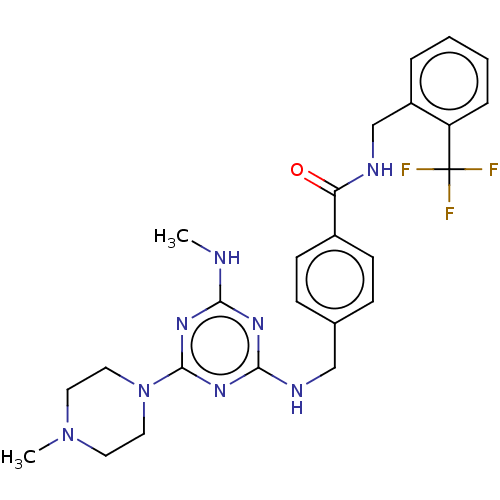
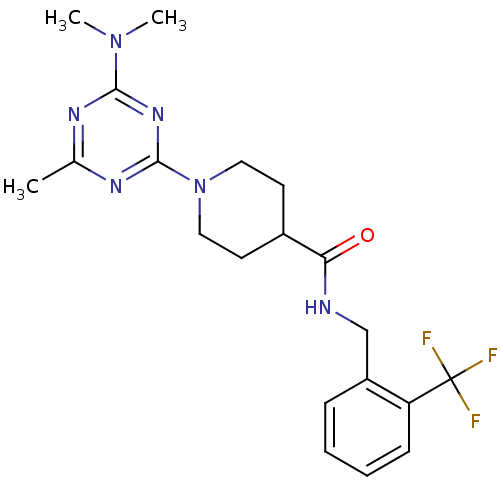
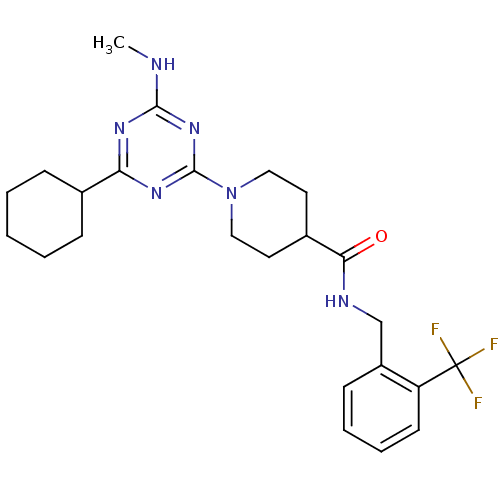
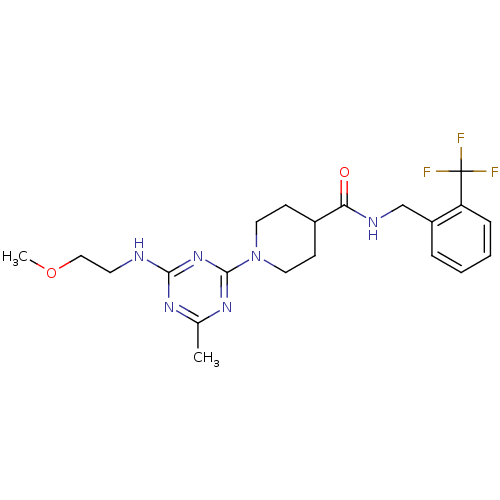
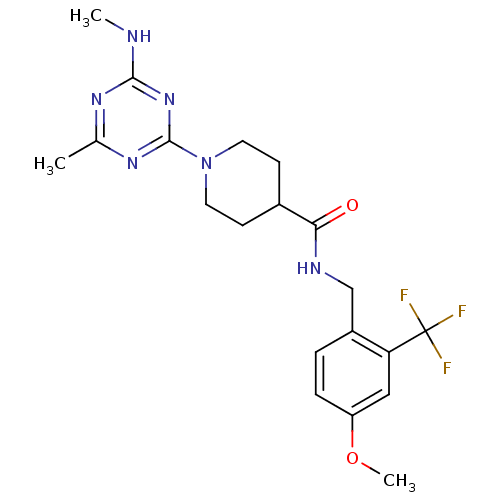
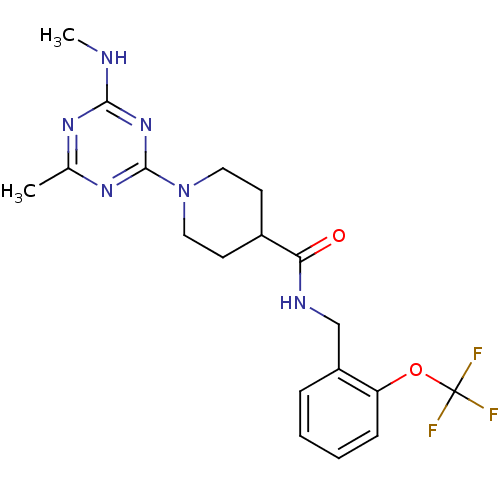
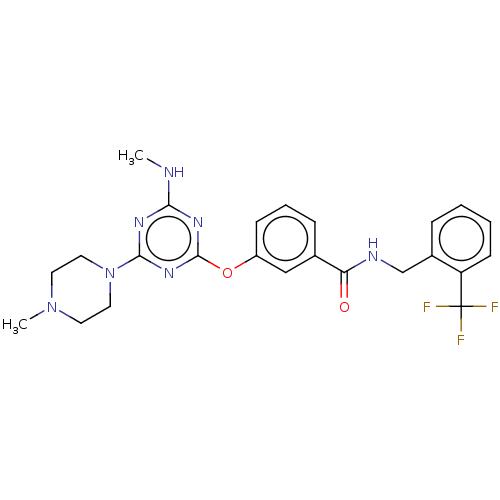
Affinity DataKi: 0.300nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 40nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 79nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 130nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 630nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 630nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.60E+3nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2.50E+3nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of rat soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prior ...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi...More data for this Ligand-Target Pair