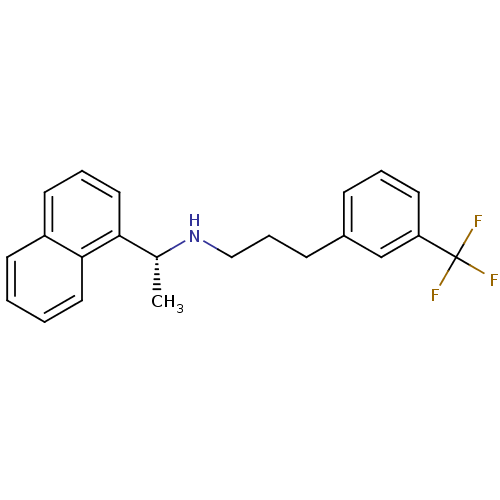
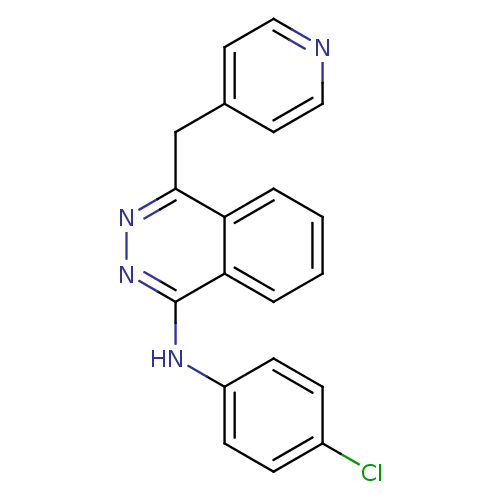
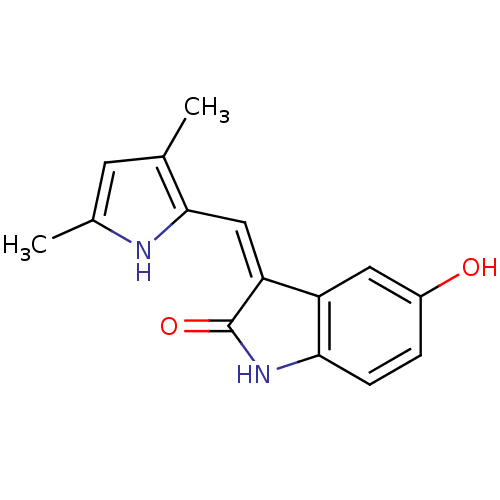
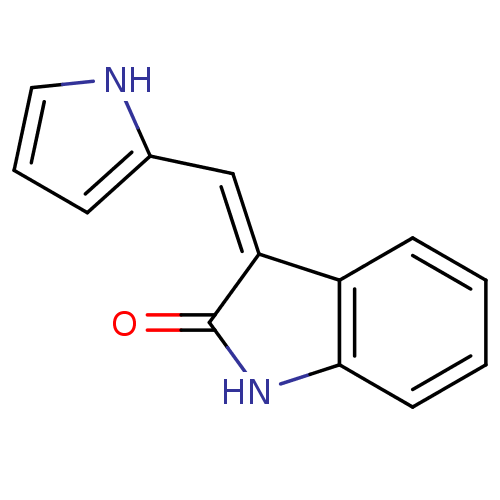
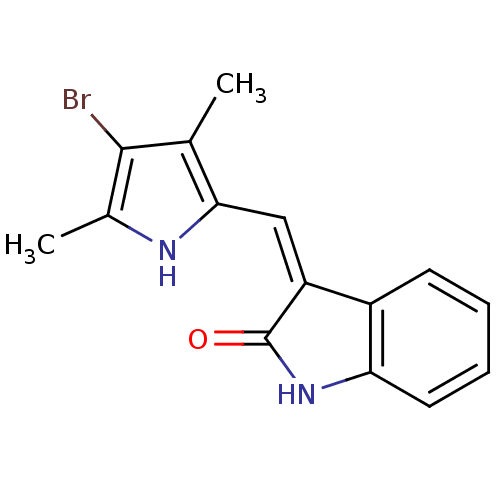
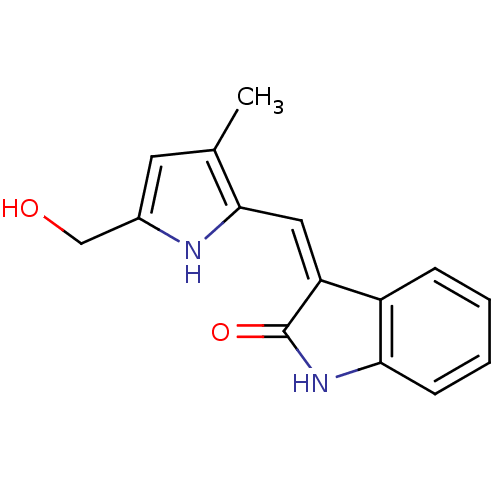
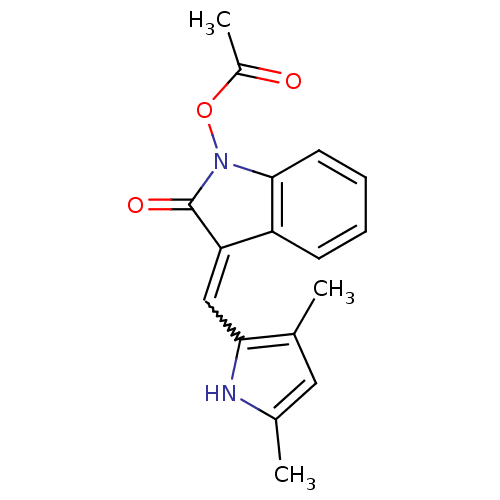
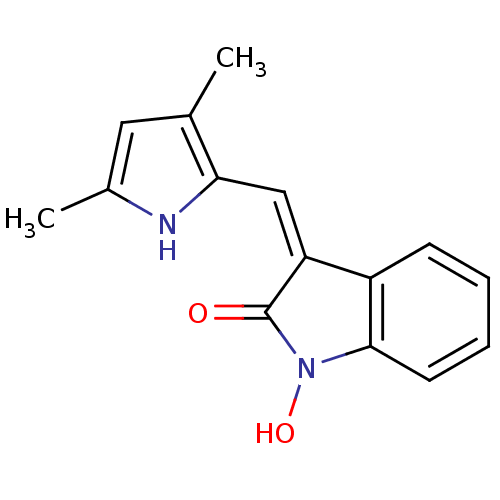
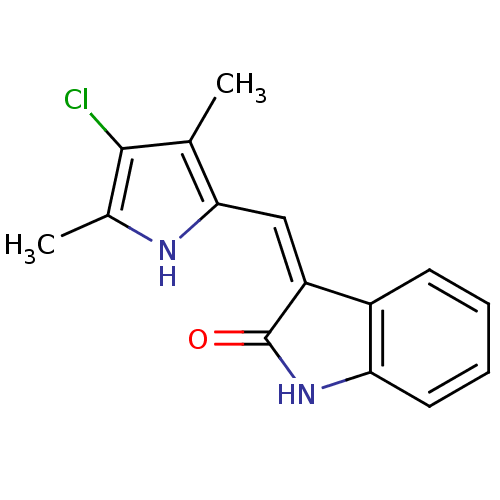
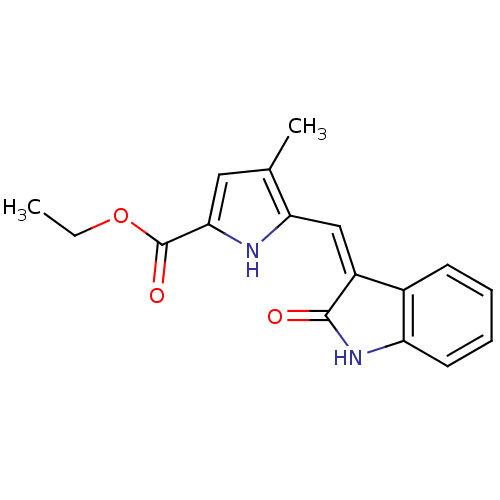
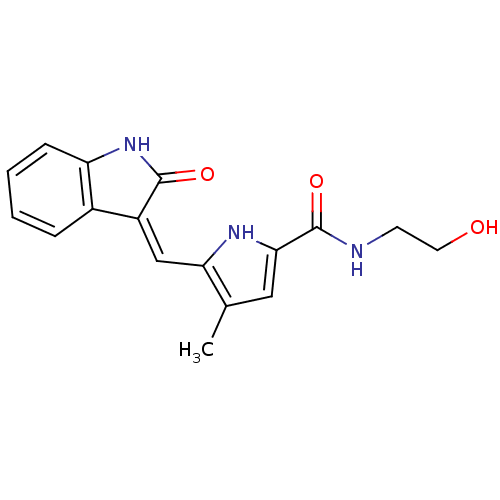
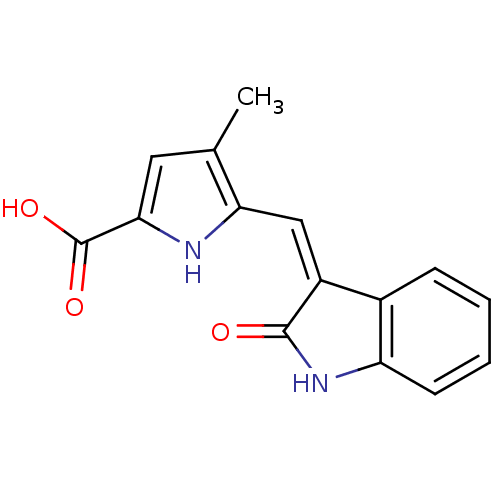
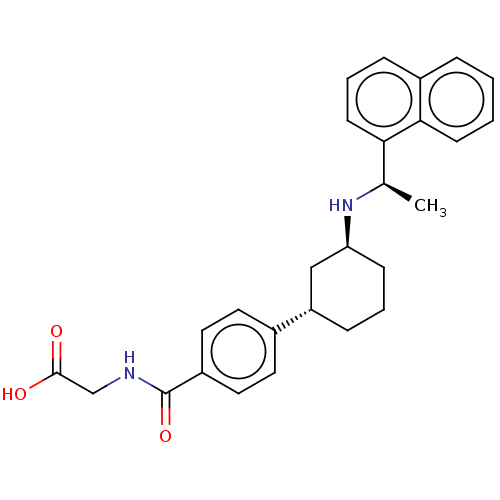
Affinity DataIC50: 50nMpH: 7.4 T: 2°CAssay Description:Incubations were conducted in 96 well microtiter plates based on a method described by BD Biosciences. To the first well in each row, a NADPH regener...More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Leo Pharma
Curated by ChEMBL
Leo Pharma
Curated by ChEMBL
Affinity DataIC50: 63nMAssay Description:In vitro inhibition of KDRMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Leo Pharma
Curated by ChEMBL
Leo Pharma
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:In vitro inhibition of KDRMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Leo Pharma
Curated by ChEMBL
Leo Pharma
Curated by ChEMBL
Affinity DataIC50: 140nMAssay Description:In vitro inhibition of KDRMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Leo Pharma
Curated by ChEMBL
Leo Pharma
Curated by ChEMBL
Affinity DataIC50: 230nMAssay Description:In vitro inhibition of KDRMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Leo Pharma
Curated by ChEMBL
Leo Pharma
Curated by ChEMBL
Affinity DataIC50: 320nMAssay Description:In vitro inhibition of KDRMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Leo Pharma
Curated by ChEMBL
Leo Pharma
Curated by ChEMBL
Affinity DataIC50: 320nMAssay Description:In vitro inhibition of KDRMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Leo Pharma
Curated by ChEMBL
Leo Pharma
Curated by ChEMBL
Affinity DataIC50: 400nMAssay Description:In vitro inhibition of KDRMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Leo Pharma
Curated by ChEMBL
Leo Pharma
Curated by ChEMBL
Affinity DataIC50: 500nMAssay Description:In vitro inhibition of KDRMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Leo Pharma
Curated by ChEMBL
Leo Pharma
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:In vitro inhibition of KDRMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Leo Pharma
Curated by ChEMBL
Leo Pharma
Curated by ChEMBL
Affinity DataIC50: 8.00E+3nMAssay Description:In vitro inhibition of KDRMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMpH: 7.4 T: 2°CAssay Description:Incubations were conducted in 96 well microtiter plates based on a method described by BD Biosciences. To the first well in each row, a NADPH regener...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMpH: 7.4 T: 2°CAssay Description:Incubations were conducted in 96 well microtiter plates based on a method described by BD Biosciences. To the first well in each row, a NADPH regener...More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Leo Pharma
Curated by ChEMBL
Leo Pharma
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:In vitro inhibition of KDRMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Leo Pharma
Curated by ChEMBL
Leo Pharma
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:In vitro inhibition of KDRMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMpH: 7.4 T: 2°CAssay Description:Incubations were conducted in 96 well microtiter plates based on a method described by BD Biosciences. To the first well in each row, a NADPH regener...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+4nMpH: 7.4 T: 2°CAssay Description:Incubations were conducted in 96 well microtiter plates based on a method described by BD Biosciences. To the first well in each row, a NADPH regener...More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMpH: 7.4 T: 2°CAssay Description:Incubations were conducted in 96 well microtiter plates based on a method described by BD Biosciences. To the first well in each row, a NADPH regener...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMpH: 7.4 T: 2°CAssay Description:Incubations were conducted in 96 well microtiter plates based on a method described by BD Biosciences. To the first well in each row, a NADPH regener...More data for this Ligand-Target Pair
Affinity DataIC50: 7.90E+4nMpH: 7.4 T: 2°CAssay Description:Incubations were conducted in 96 well microtiter plates based on a method described by BD Biosciences. To the first well in each row, a NADPH regener...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMpH: 7.4 T: 2°CAssay Description:Incubations were conducted in 96 well microtiter plates based on a method described by BD Biosciences. To the first well in each row, a NADPH regener...More data for this Ligand-Target Pair