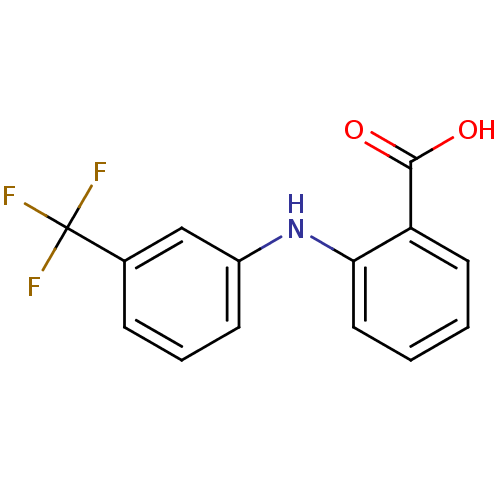
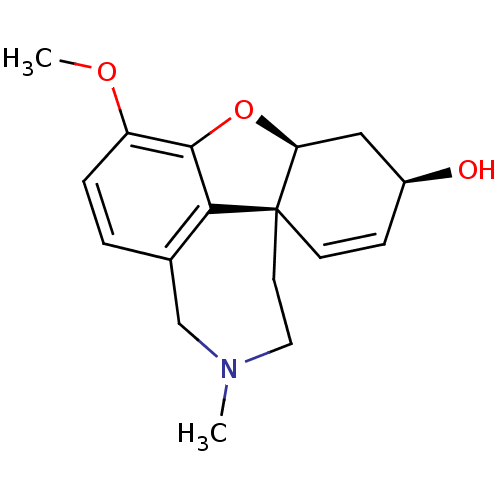
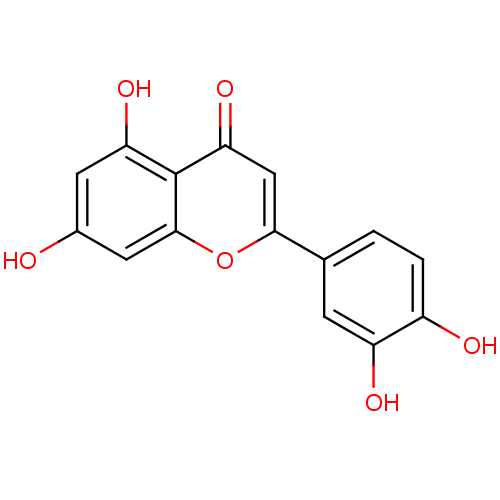
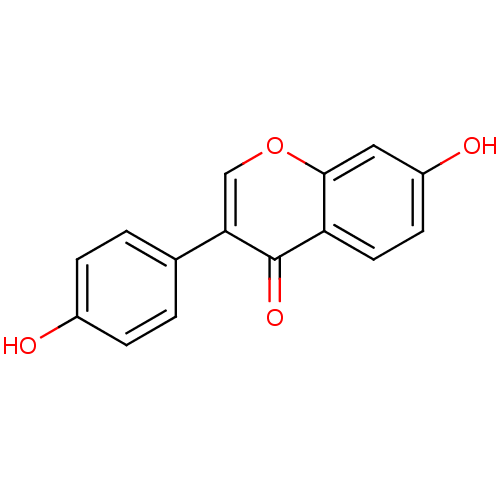
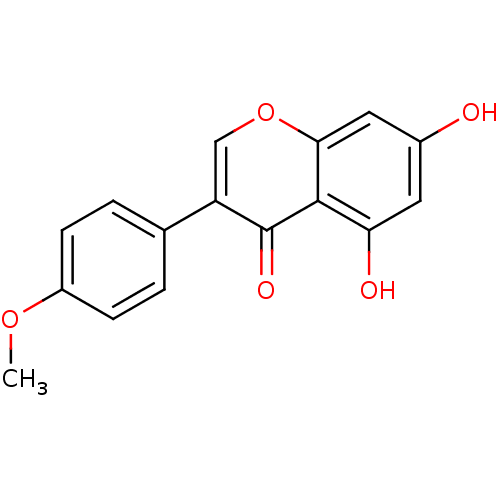
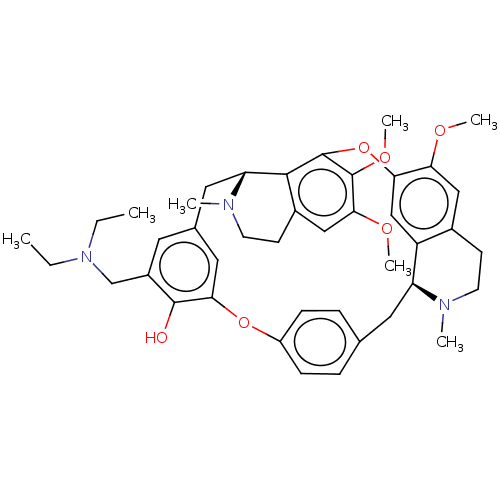
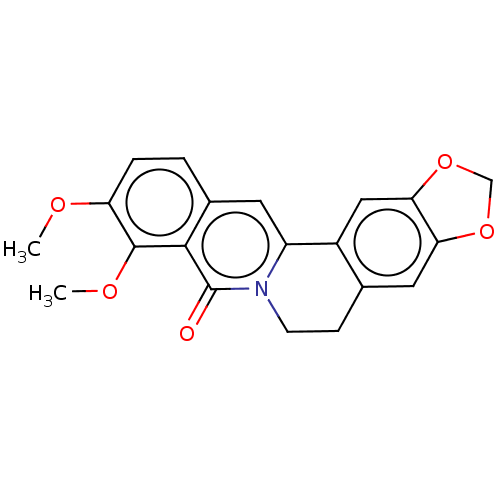
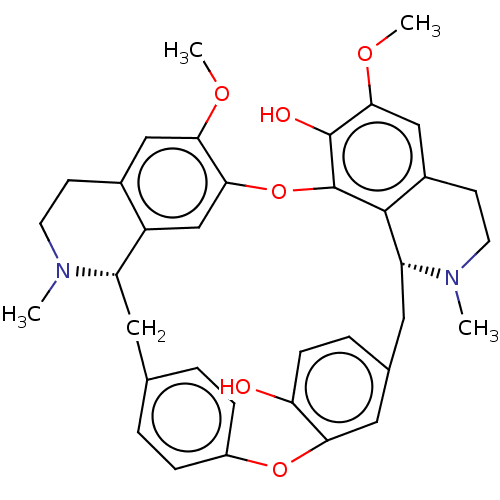
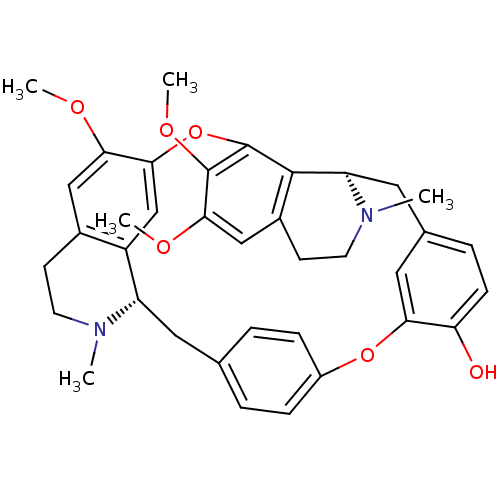
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
University Of Hradec Kralove
Curated by ChEMBL
University Of Hradec Kralove
Curated by ChEMBL
Affinity DataKi: 6.00E+3nMAssay Description:Non-competitive inhibition of recombinant human N-terminal His6-tagged AKR1B10 expressed in Escherichia coli BL21 cells using all-trans-retinal as su...More data for this Ligand-Target Pair
Affinity DataKi: 6.41E+3nMAssay Description:Mixed-type inhibition of human BuChE assessed as enzyme-inhibitor complex using butyrylthiocholine iodide as substrate by Lineweaver-Burk plot analys...More data for this Ligand-Target Pair
Affinity DataKi: 3.97E+4nMAssay Description:Mixed-type inhibition of human BuChE assessed as enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrate by Lineweaver-Burk p...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:Inhibition of POP (unknown origin) using (Z)-Gly-Pro-p-nitroanilide as substrate preincubated for 5 mins followed by substrate addition and measured ...More data for this Ligand-Target Pair
Affinity DataIC50: 33nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 700nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by Ellman's methodMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
University Of Hradec Kralove
Curated by ChEMBL
University Of Hradec Kralove
Curated by ChEMBL
Affinity DataIC50: 760nMAssay Description:Inhibition of recombinant human N-terminal His6-tagged AKR1B10 expressed in Escherichia coli BL21 (DE3) pLysS cells by pyridine-3-aldehyde reductase ...More data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by Ellman's methodMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
University Of Hradec Kralove
Curated by ChEMBL
University Of Hradec Kralove
Curated by ChEMBL
Affinity DataIC50: 3.20E+3nMAssay Description:Inhibition of recombinant N-terminal His6-tagged AKR1B1 (unknown origin) expressed in Escherichia coli BL21 cells using all-trans-retinal as substrat...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
University Of Hradec Kralove
Curated by ChEMBL
University Of Hradec Kralove
Curated by ChEMBL
Affinity DataIC50: 6.60E+3nMAssay Description:Inhibition of recombinant human N-terminal His6-tagged AKR1B10 expressed in Escherichia coli BL21 cells using all-trans-retinal as substrate incubate...More data for this Ligand-Target Pair
Affinity DataIC50: 6.90E+3nMAssay Description:Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by Ellman's methodMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
University Of Hradec Kralove
Curated by ChEMBL
University Of Hradec Kralove
Curated by ChEMBL
Affinity DataIC50: 8.30E+3nMAssay Description:Inhibition of recombinant human N-terminal His6-tagged AKR1B10 expressed in Escherichia coli BL21 cells using all-trans-retinal as substrate incubate...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
University Of Hradec Kralove
Curated by ChEMBL
University Of Hradec Kralove
Curated by ChEMBL
Affinity DataIC50: 9.20E+3nMAssay Description:Inhibition of recombinant human N-terminal His6-tagged AKR1B10 expressed in Escherichia coli BL21 cells using all-trans-retinal as substrate incubate...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
University Of Hradec Kralove
Curated by ChEMBL
University Of Hradec Kralove
Curated by ChEMBL
Affinity DataIC50: 1.19E+4nMAssay Description:Inhibition of recombinant N-terminal His6-tagged AKR1B1 (unknown origin) expressed in Escherichia coli BL21 cells using all-trans-retinal as substrat...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
University Of Hradec Kralove
Curated by ChEMBL
University Of Hradec Kralove
Curated by ChEMBL
Affinity DataIC50: 1.23E+4nMAssay Description:Inhibition of recombinant N-terminal His6-tagged AKR1B1 (unknown origin) expressed in Escherichia coli BL21 cells using all-trans-retinal as substrat...More data for this Ligand-Target Pair
Affinity DataIC50: 3.07E+4nMAssay Description:Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by Ellman's methodMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
University Of Hradec Kralove
Curated by ChEMBL
University Of Hradec Kralove
Curated by ChEMBL
Affinity DataIC50: 3.22E+4nMAssay Description:Inhibition of recombinant human N-terminal His6-tagged AKR1B10 expressed in Escherichia coli BL21 cells using all-trans-retinal as substrate incubate...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
University Of Hradec Kralove
Curated by ChEMBL
University Of Hradec Kralove
Curated by ChEMBL
Affinity DataIC50: 3.61E+4nMAssay Description:Inhibition of recombinant human N-terminal His6-tagged AKR1B10 expressed in Escherichia coli BL21 cells using all-trans-retinal as substrate incubate...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
University Of Hradec Kralove
Curated by ChEMBL
University Of Hradec Kralove
Curated by ChEMBL
Affinity DataIC50: 3.71E+4nMAssay Description:Inhibition of recombinant human N-terminal His6-tagged AKR1B10 expressed in Escherichia coli BL21 cells using daunorubicin as substrate incubated for...More data for this Ligand-Target Pair
Affinity DataIC50: 4.23E+4nMAssay Description:Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.60E+4nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.70E+4nMAssay Description:Inhibition of POP (unknown origin) using (Z)-Gly-Pro-p-nitroanilide as substrate preincubated for 5 mins followed by substrate addition and measured ...More data for this Ligand-Target Pair
Affinity DataIC50: 6.80E+4nMAssay Description:Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.80E+4nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.80E+4nMAssay Description:Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by Ellman's methodMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
University Of Hradec Kralove
Curated by ChEMBL
University Of Hradec Kralove
Curated by ChEMBL
Affinity DataIC50: 9.60E+4nMAssay Description:Inhibition of recombinant human N-terminal His6-tagged AKR1B10 expressed in Escherichia coli BL21 cells using all-trans-retinal as substrate incubate...More data for this Ligand-Target Pair
Affinity DataIC50: 9.70E+4nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by Ellman's methodMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
University Of Hradec Kralove
Curated by ChEMBL
University Of Hradec Kralove
Curated by ChEMBL
Affinity DataIC50: 1.08E+5nMAssay Description:Inhibition of recombinant human N-terminal His6-tagged AKR1B10 expressed in Escherichia coli BL21 cells using daunorubicin as substrate incubated for...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
University Of Hradec Kralove
Curated by ChEMBL
University Of Hradec Kralove
Curated by ChEMBL
Affinity DataIC50: 1.27E+5nMAssay Description:Inhibition of recombinant N-terminal His6-tagged AKR1B1 (unknown origin) expressed in Escherichia coli BL21 cells using all-trans-retinal as substrat...More data for this Ligand-Target Pair
Affinity DataIC50: 1.42E+5nMAssay Description:Inhibition of POP (unknown origin) using (Z)-Gly-Pro-p-nitroanilide as substrate preincubated for 5 mins followed by substrate addition and measured ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.45E+5nMAssay Description:Inhibition of POP (unknown origin)More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
University Of Hradec Kralove
Curated by ChEMBL
University Of Hradec Kralove
Curated by ChEMBL
Affinity DataIC50: 1.60E+5nMAssay Description:Inhibition of recombinant human N-terminal His6-tagged AKR1B10 expressed in Escherichia coli BL21 cells using daunorubicin as substrate incubated for...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
University Of Hradec Kralove
Curated by ChEMBL
University Of Hradec Kralove
Curated by ChEMBL
Affinity DataIC50: 1.62E+5nMAssay Description:Inhibition of recombinant N-terminal His6-tagged AKR1B1 (unknown origin) expressed in Escherichia coli BL21 cells using all-trans-retinal as substrat...More data for this Ligand-Target Pair
Affinity DataIC50: 1.89E+5nMAssay Description:Inhibition of POP (unknown origin) using (Z)-Gly-Pro-p-nitroanilide as substrate preincubated for 5 mins followed by substrate addition and measured ...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+5nMAssay Description:Inhibition of POP (unknown origin) using (Z)-Gly-Pro-p-nitroanilide as substrate preincubated for 5 mins followed by substrate addition and measured ...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+5nMAssay Description:Inhibition of POP (unknown origin) using (Z)-Gly-Pro-p-nitroanilide as substrate preincubated for 5 mins followed by substrate addition and measured ...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+5nMAssay Description:Inhibition of POP (unknown origin) using (Z)-Gly-Pro-p-nitroanilide as substrate preincubated for 5 mins followed by substrate addition and measured ...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+5nMAssay Description:Inhibition of POP (unknown origin) using (Z)-Gly-Pro-p-nitroanilide as substrate preincubated for 5 mins followed by substrate addition and measured ...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+5nMAssay Description:Inhibition of POP (unknown origin) using (Z)-Gly-Pro-p-nitroanilide as substrate preincubated for 5 mins followed by substrate addition and measured ...More data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)