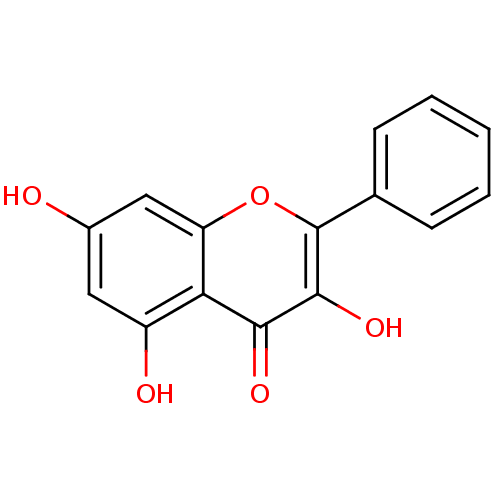
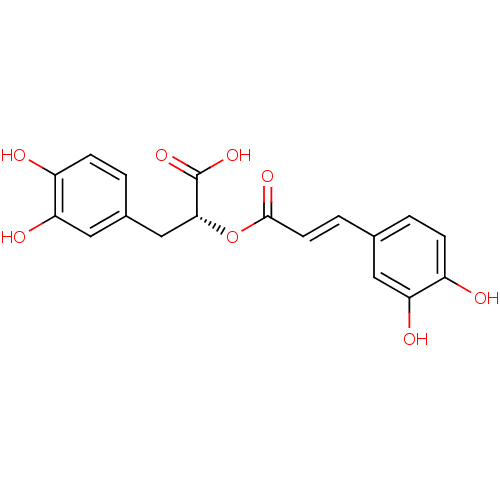
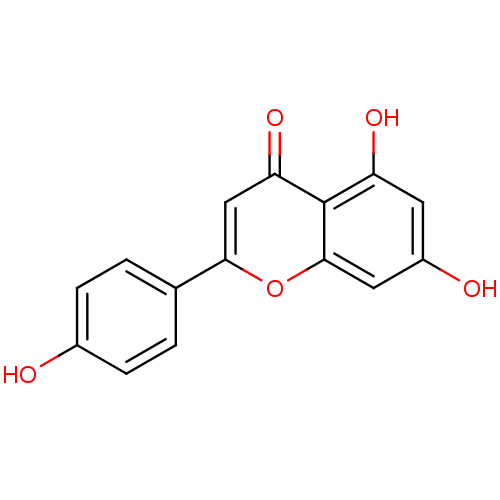
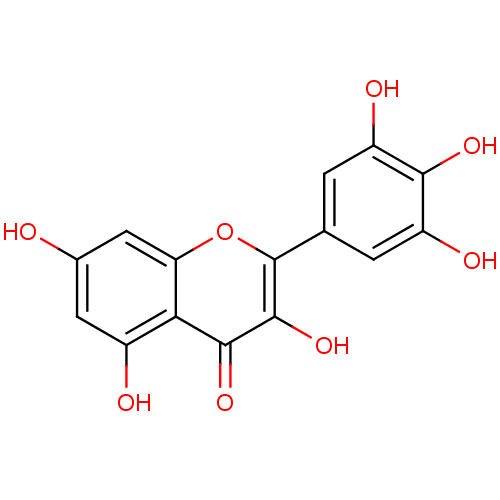
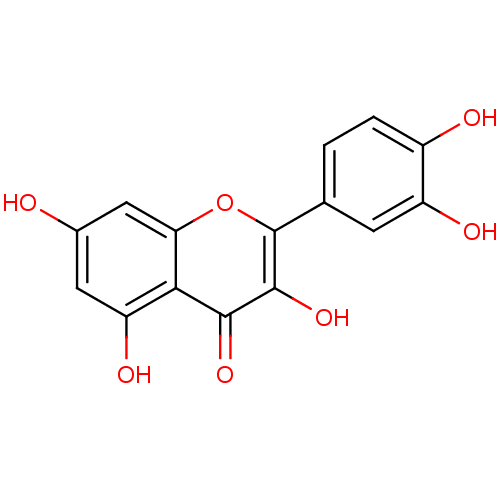
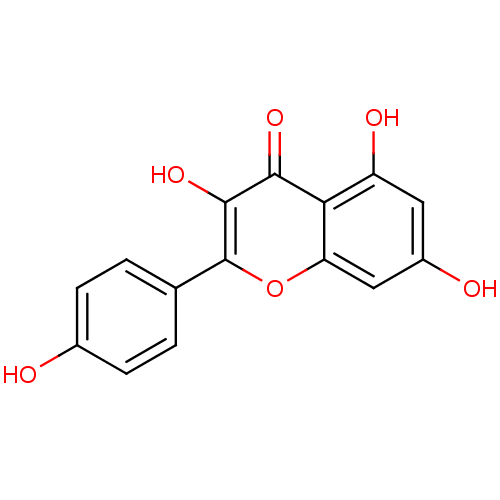
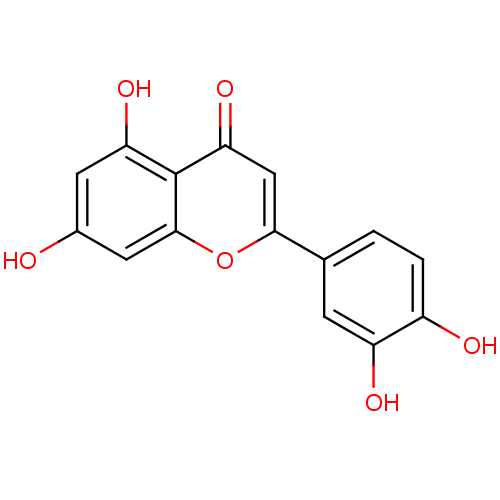
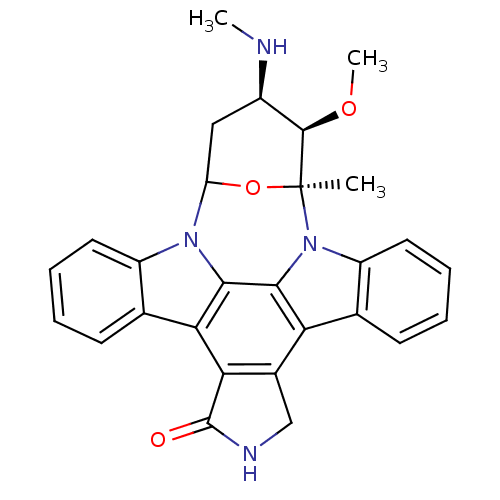
TargetCholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 6.90E+3nMAssay Description:Inhibition of human plasma BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataKi: 7.50E+3nMAssay Description:Inhibition of human Fyn expressed in Sf9 cells at 10 umol/L by ELISAMore data for this Ligand-Target Pair
Affinity DataKi: 1.02E+4nMAssay Description:Inhibition of human Fyn expressed in Sf9 cells at 30 umol/L by ELISAMore data for this Ligand-Target Pair
TargetCholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 3.74E+4nMAssay Description:Inhibition of human plasma BChE by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 3.78E+4nMAssay Description:Inhibition of human recombinant AChE by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 3.83E+4nMAssay Description:Inhibition of human recombinant AChE by Ellman's methodMore data for this Ligand-Target Pair
TargetCholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 4.31E+4nMAssay Description:Inhibition of human plasma BChE by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 6.58E+4nMAssay Description:Inhibition of human recombinant AChE by Ellman's methodMore data for this Ligand-Target Pair
TargetCholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 6.80E+4nMAssay Description:Inhibition of human plasma BChE by Ellman's methodMore data for this Ligand-Target Pair
TargetCholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 7.10E+4nMAssay Description:Inhibition of human plasma BChE by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 8.56E+4nMAssay Description:Inhibition of human recombinant AChE by Ellman's methodMore data for this Ligand-Target Pair
TargetCholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 9.00E+4nMAssay Description:Inhibition of human plasma BChE by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 9.28E+4nMAssay Description:Inhibition of human recombinant AChE by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 1.00E+5nMAssay Description:Inhibition of human recombinant AChE by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 1.22E+5nMAssay Description:Inhibition of human recombinant AChE by Ellman's methodMore data for this Ligand-Target Pair
TargetCholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 1.66E+5nMAssay Description:Inhibition of human plasma BChE by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: >3.00E+5nMAssay Description:Inhibition of human recombinant AChE by Ellman's methodMore data for this Ligand-Target Pair
TargetCholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 5.01E+5nMAssay Description:Inhibition of human plasma BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human Fyn expressed in Sf9 cells after 1 min by ELISA in presence of 1 umol/L ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Inhibition of human Fyn expressed in Sf9 cells after 20 mins by ELISA in presence of 1 umol/L ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:Inhibition of human Fyn expressed in Sf9 cells after 1 min by ELISA in presence of 10 umol/L ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 83nMAssay Description:Inhibition of human Fyn expressed in Sf9 cells after 20 mins by ELISA in presence of 10 umol/L ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 270nMAssay Description:Inhibition of human Fyn expressed in Sf9 cells after 1 min by ELISA in presence of 100 umol/L ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:Inhibition of human recombinant CYP3A4 using DEF as substrate preincubated for 5 to 10 mins before substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: 510nMAssay Description:Inhibition of human Fyn expressed in Sf9 cells after 20 mins by ELISA in presence of 100 umol/L ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 700nMAssay Description:Inhibition of human recombinant CYP3A4 using DEF as substrate preincubated for 5 to 10 mins before substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: 700nMAssay Description:Inhibition of human recombinant CYP3A4 using DEF as substrate preincubated for 5 to 10 mins before substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of human recombinant CYP3A4 using DEF as substrate preincubated for 5 to 10 mins before substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of human recombinant CYP3A4 using DEF as substrate preincubated for 5 to 10 mins before substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of human Fyn expressed in Sf9 cells after 20 mins by ELISA in presence of 1 umol/L ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+3nMAssay Description:Inhibition of human recombinant CYP3A4 using DEF as substrate preincubated for 5 to 10 mins before substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of human recombinant CYP3A4 using DEF as substrate preincubated for 5 to 10 mins before substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: 2.90E+3nMAssay Description:Inhibition of human recombinant CYP3A4 using 7-BQ as substrate preincubated for 5 to 10 mins before substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of human Fyn expressed in Sf9 cells after 20 mins by ELISA in presence of 10 umol/L ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70E+3nMAssay Description:Inhibition of human recombinant CYP3A4 using 7-BQ as substrate preincubated for 5 to 10 mins before substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: 5.70E+3nMAssay Description:Inhibition of human recombinant CYP3A4 using 7-BQ as substrate preincubated for 5 to 10 mins before substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: 7.80E+3nMAssay Description:Inhibition of human recombinant CYP3A4 using DEF as substrate preincubated for 5 to 10 mins before substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: 7.90E+3nMAssay Description:Inhibition of human recombinant CYP3A4 using DEF as substrate preincubated for 5 to 10 mins before substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of human recombinant CYP3A4 using 7-BQ as substrate preincubated for 5 to 10 mins before substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: 9.80E+3nMAssay Description:Inhibition of human recombinant CYP3A4 using 7-BQ as substrate preincubated for 5 to 10 mins before substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human recombinant CYP3A4 using 7-BQ as substrate preincubated for 5 to 10 mins before substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human recombinant CYP3A4 using DEF as substrate preincubated for 5 to 10 mins before substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human recombinant CYP3A4 using 7-BQ as substrate preincubated for 5 to 10 mins before substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human recombinant CYP3A4 using 7-BQ as substrate preincubated for 5 to 10 mins before substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human recombinant CYP3A4 using 7-BQ as substrate preincubated for 5 to 10 mins before substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human recombinant CYP3A4 using 7-BQ as substrate preincubated for 5 to 10 mins before substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human recombinant CYP3A4 using 7-BQ as substrate preincubated for 5 to 10 mins before substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+4nMAssay Description:Inhibition of human Fyn expressed in Sf9 cells after 20 mins by ELISA in presence of 100 umol/L ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+4nMAssay Description:Inhibition of human Fyn expressed in Sf9 cells after 1 min by ELISA in presence of 1 umol/L ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 6.30E+4nMAssay Description:Inhibition of human Fyn expressed in Sf9 cells after 1 min by ELISA in presence of 100 umol/L ATPMore data for this Ligand-Target Pair