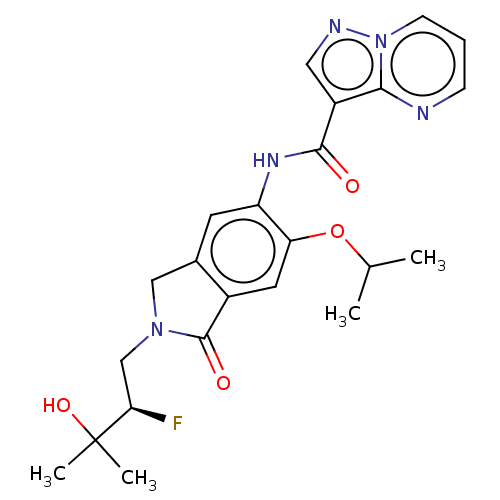
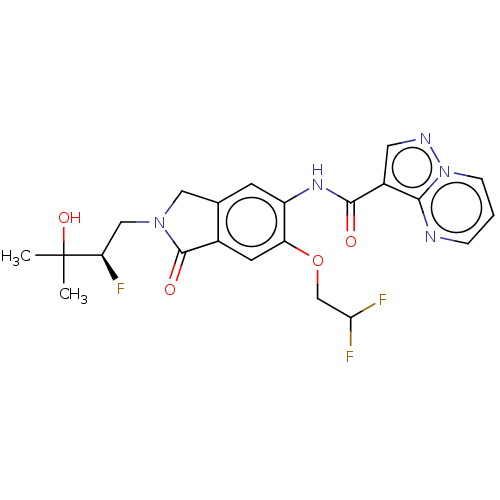
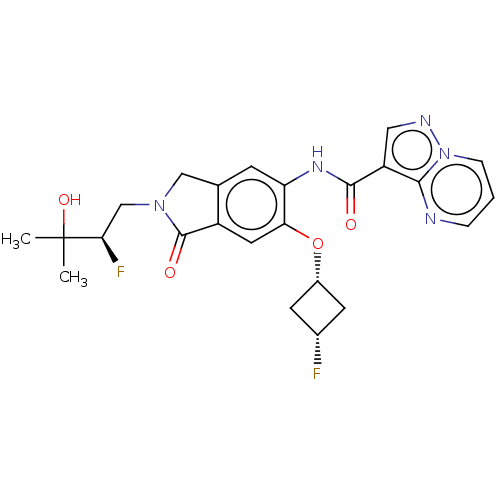
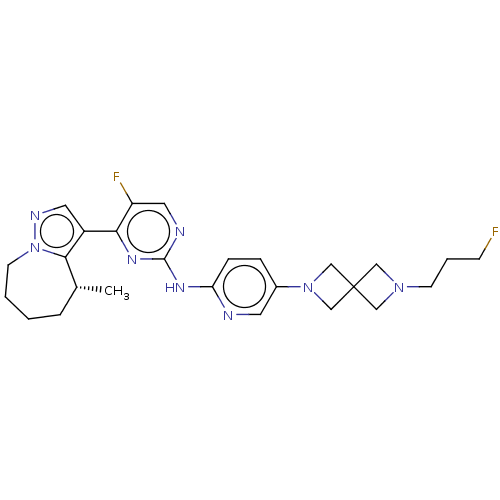
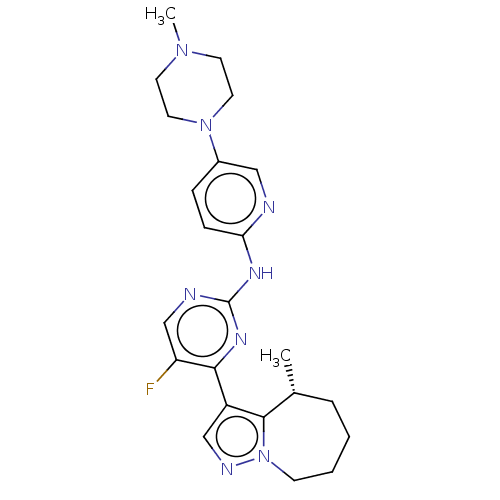
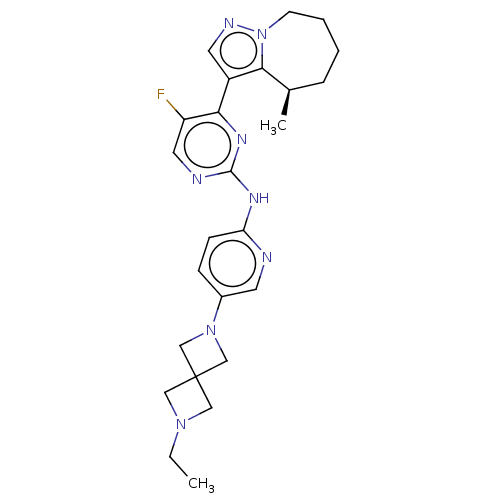
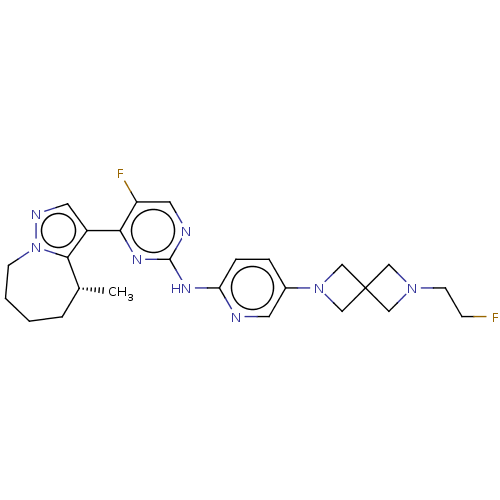
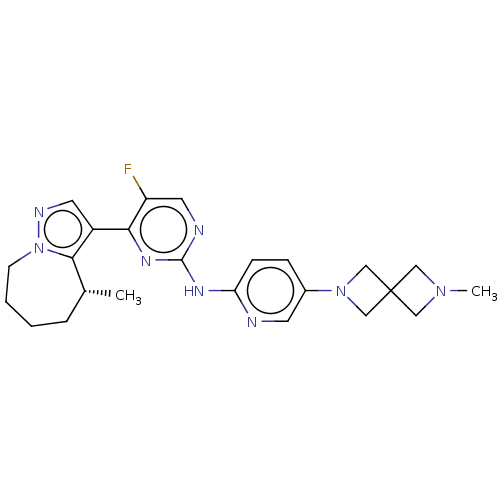
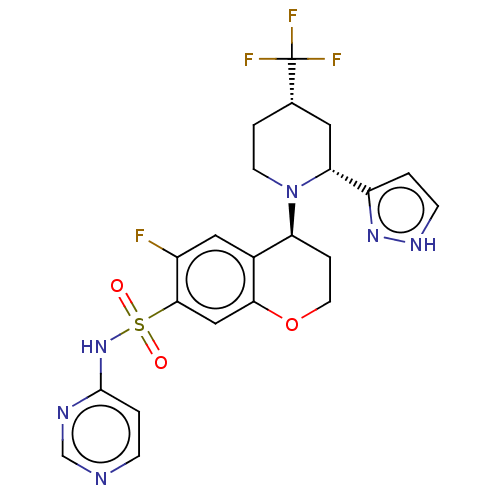
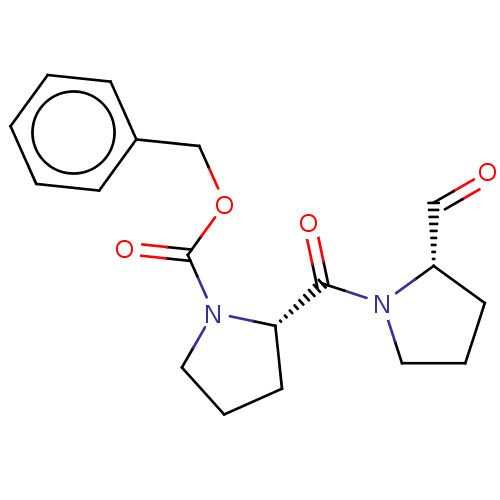
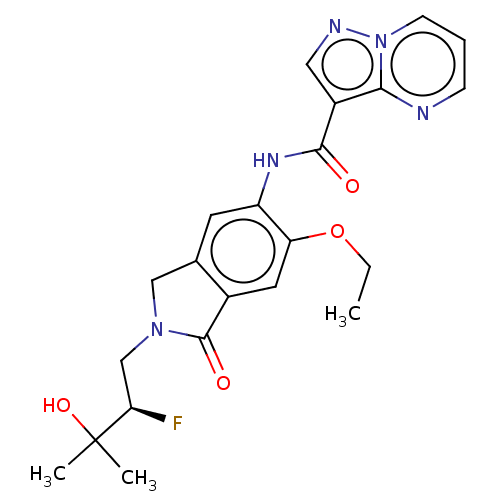
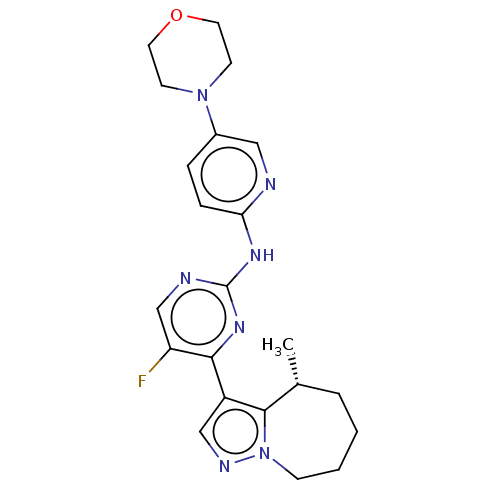
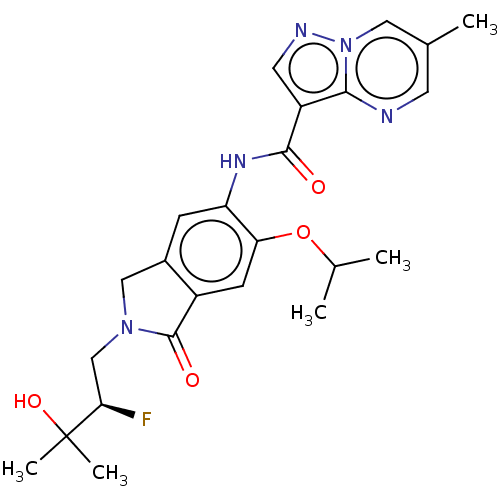
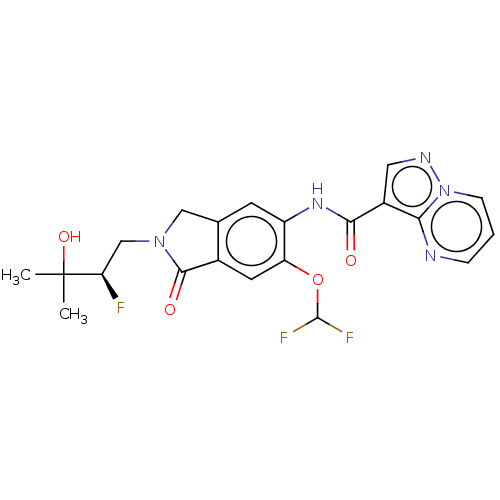
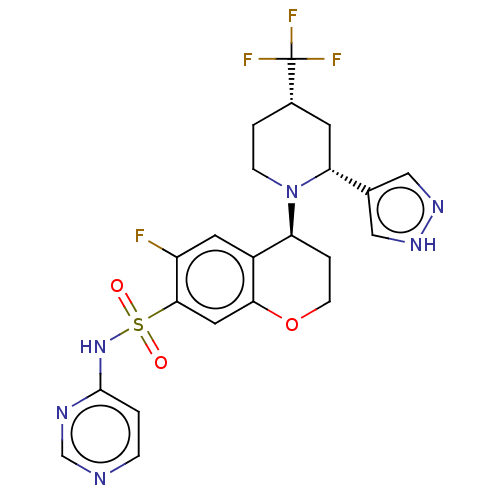
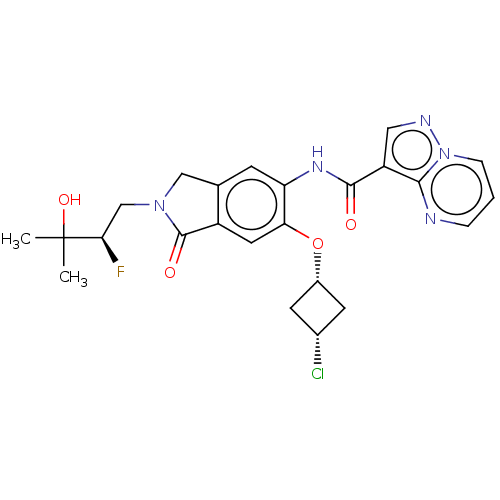
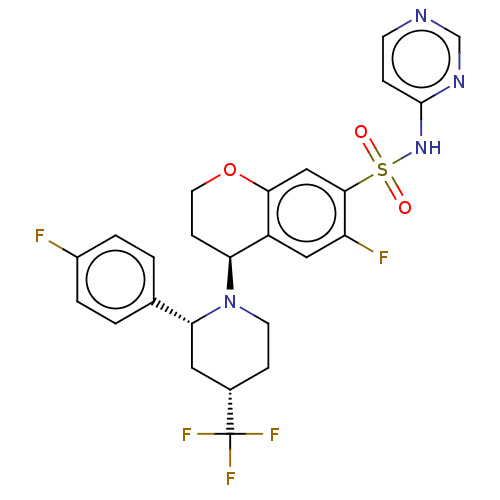
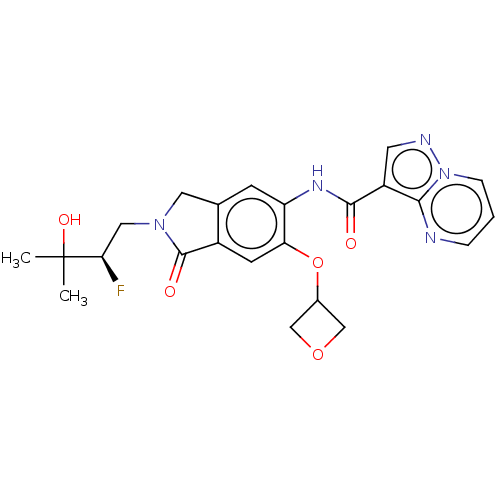
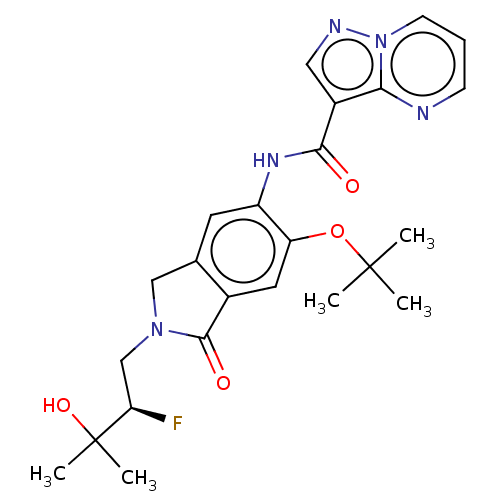
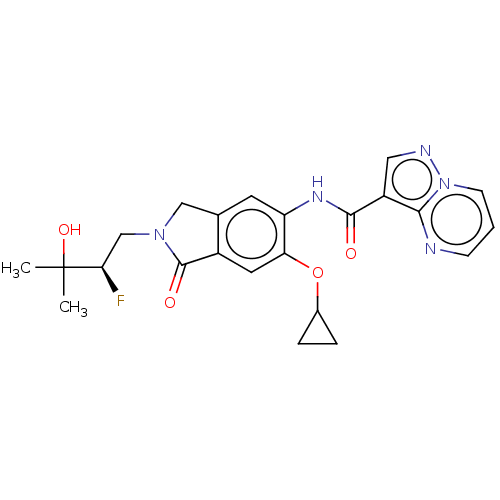
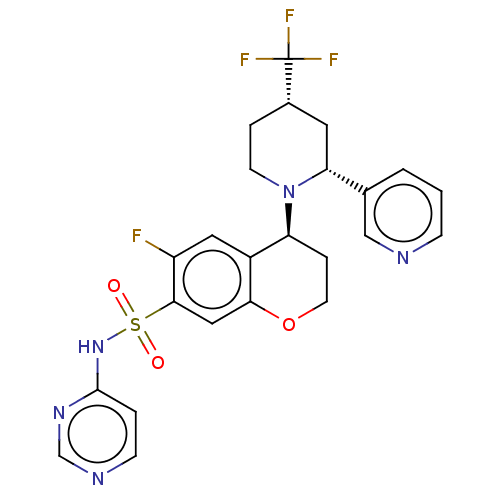
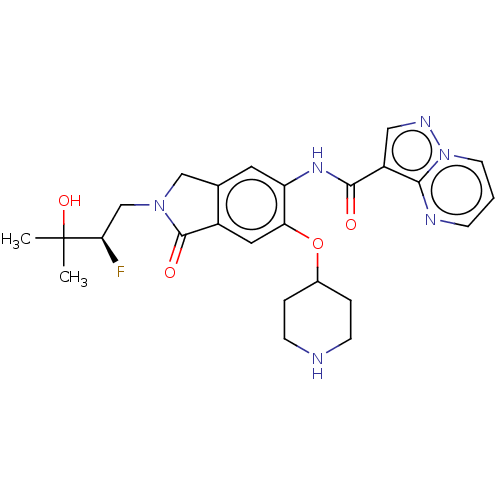
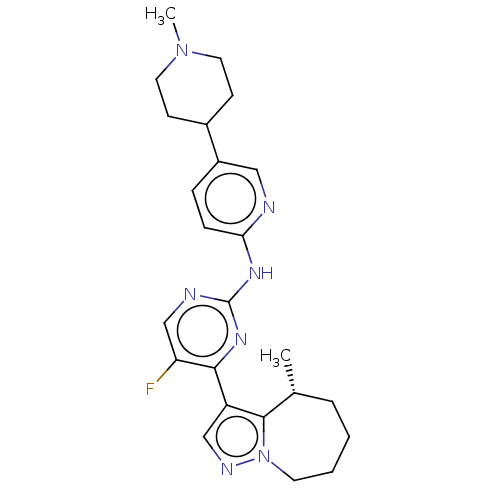
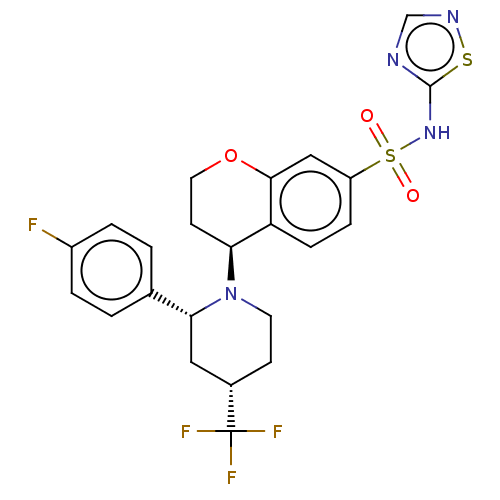
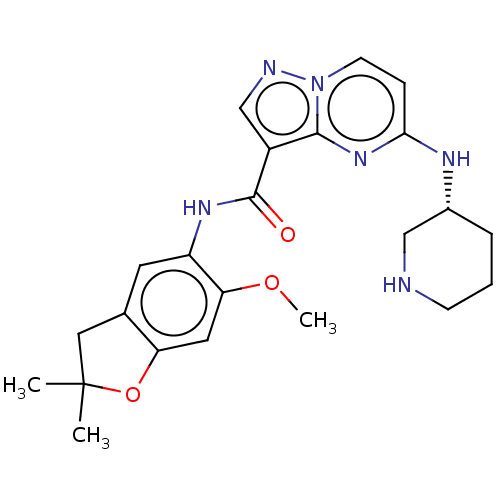
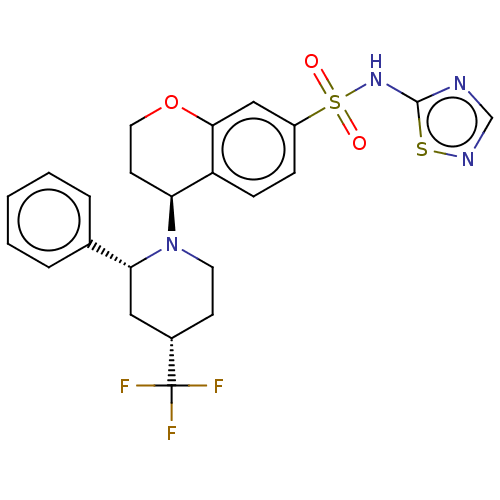
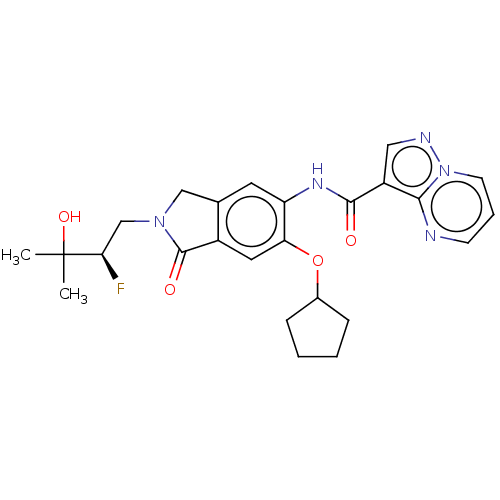
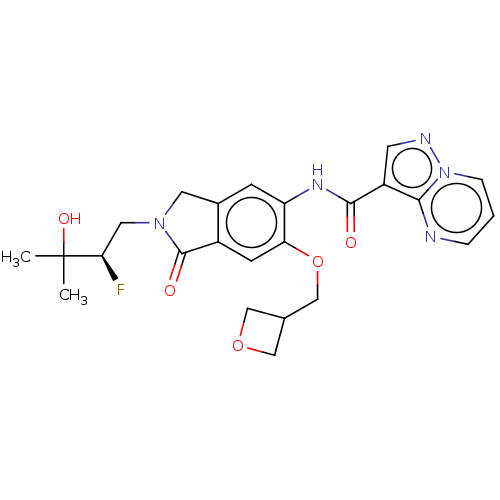
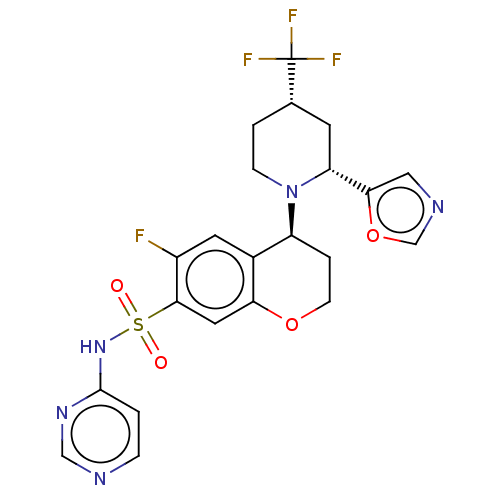
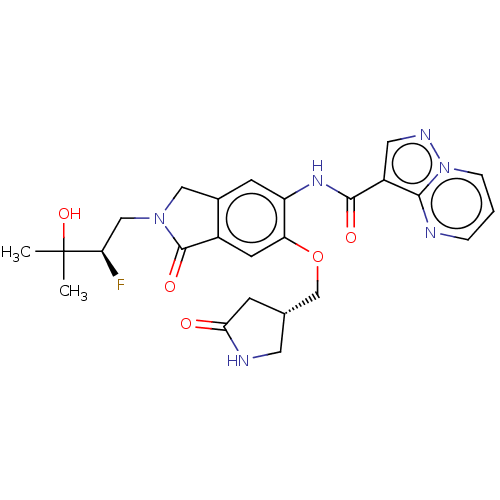
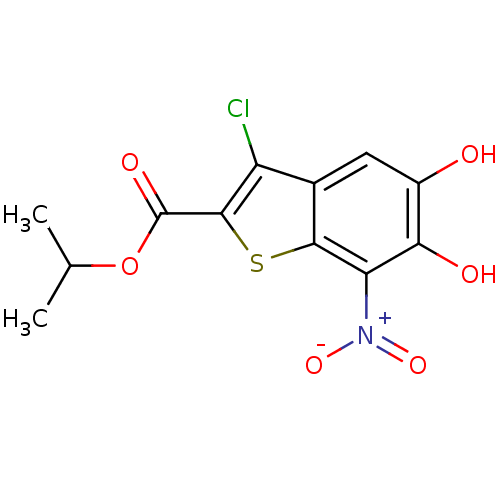
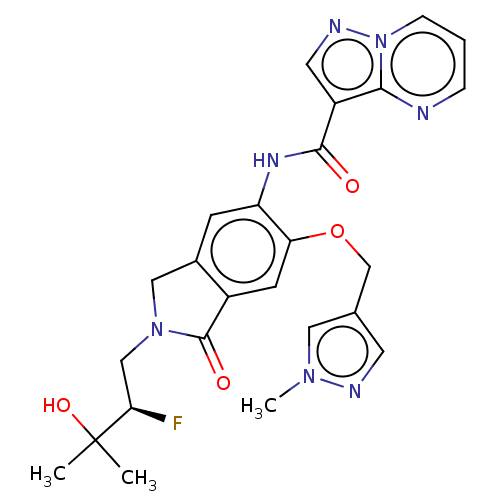
Affinity DataKi: 0.0200nMAssay Description:Inhibition of POP in Han/Wistar rat brain using Suc-Gly-Pro-AMC substrate incubated for 60 minsMore data for this Ligand-Target Pair
Affinity DataKi: 0.0600nMAssay Description:Inhibition of poricne brain POP in expressed in Escherichia coli TOP10 competent cells pre-incubated for 2 hrs before Z-Gly-Pro-AMC substrate additio...More data for this Ligand-Target Pair
Affinity DataKi: 0.170nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 0.260nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 0.280nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 4/G1/S-specific cyclin-D3(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: <0.300nMAssay Description:Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 4/G1/S-specific cyclin-D3(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: <0.300nMAssay Description:Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 4/G1/S-specific cyclin-D3(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: <0.300nMAssay Description:Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 4/G1/S-specific cyclin-D3(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: <0.300nMAssay Description:Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 4/G1/S-specific cyclin-D3(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: <0.300nMAssay Description:Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression...More data for this Ligand-Target Pair
Affinity DataKi: 0.320nMAssay Description:Displacement of [3H]GX-545 from full length human Nav1.7 VSD4 domain expressed in HEK cell membranes measured after 20 hrs by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 0.350nMAssay Description:Inhibition of POP in bovine serum using Z-Gly-Pro-NH-Mec fluorimetric substrateMore data for this Ligand-Target Pair
Affinity DataKi: 0.370nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 4/G1/S-specific cyclin-D3(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.400nMAssay Description:Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 4/G1/S-specific cyclin-D3(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.400nMAssay Description:Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression...More data for this Ligand-Target Pair
Affinity DataKi: 0.420nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 0.490nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 0.510nMAssay Description:Displacement of [3H]GX-545 from full length human Nav1.7 VSD4 domain expressed in HEK cell membranes measured after 20 hrs by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 0.510nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 0.530nMAssay Description:Displacement of [3H]GX-545 from full length human Nav1.7 VSD4 domain expressed in HEK cell membranes measured after 20 hrs by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 0.590nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 0.610nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 0.630nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 0.630nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 0.670nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 0.680nMAssay Description:Displacement of [3H]GX-545 from full length human Nav1.7 VSD4 domain expressed in HEK cell membranes measured after 20 hrs by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 0.710nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 0.75nMAssay Description:Inhibition of human POP by tight binding based Morrison equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.760nMAssay Description:Displacement of [3H]GX-545 from full length human Nav1.7 VSD4 domain expressed in HEK cell membranes measured after 20 hrs by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 0.790nMAssay Description:Displacement of [3H]GX-545 from full length human Nav1.7 VSD4 domain expressed in HEK cell membranes measured after 20 hrs by liquid scintillation co...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 4/G1/S-specific cyclin-D3(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression...More data for this Ligand-Target Pair
Affinity DataKi: 0.850nMAssay Description:Displacement of [3H]GX-545 from full length human Nav1.7 VSD4 domain expressed in HEK cell membranes measured after 20 hrs by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 0.870nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 0.920nMAssay Description:Inhibition of human POP by tight binding based Morrison equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.930nMAssay Description:Displacement of [3H]GX-545 from full length human Nav1.7 VSD4 domain expressed in HEK cell membranes measured after 20 hrs by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 0.950nMAssay Description:Inhibition of human POP expressed in Escherichia coli BL21 pre-incubated for 30 mins before ZGP-pNA substrate additionMore data for this Ligand-Target Pair
Affinity DataKi: 0.970nMAssay Description:Displacement of [3H]GX-545 from full length human Nav1.7 VSD4 domain expressed in HEK cell membranes measured after 20 hrs by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 0.970nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 0.970nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Displacement of [3H]GX-545 from full length human Nav1.7 VSD4 domain expressed in HEK cell membranes measured after 20 hrs by liquid scintillation co...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 4/G1/S-specific cyclin-D3(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 4/G1/S-specific cyclin-D3(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Inhibition of human POP expressed in Escherichia coli BL21 pre-incubated for 30 mins before ZGP-pNA substrate additionMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
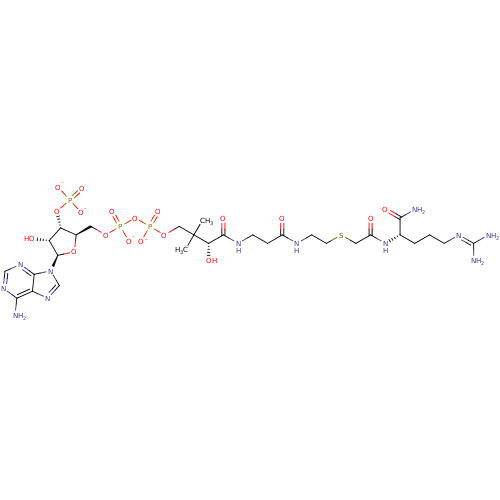
TargetCatechol O-methyltransferase(Homo sapiens (Human))
University Of Eastern Finland
Curated by ChEMBL
University Of Eastern Finland
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Inhibition of human recombinant COMT by microplate screening assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Inhibition of Enterococcus faecium AAC(6')IiMore data for this Ligand-Target Pair

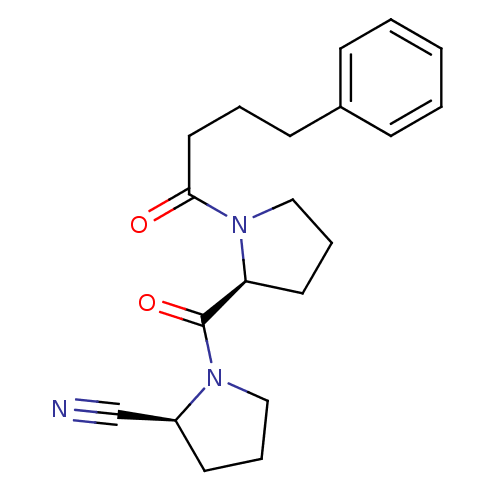
 3D Structure (crystal)
3D Structure (crystal)