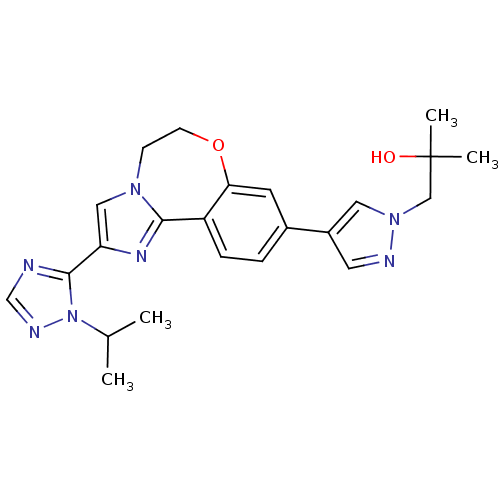
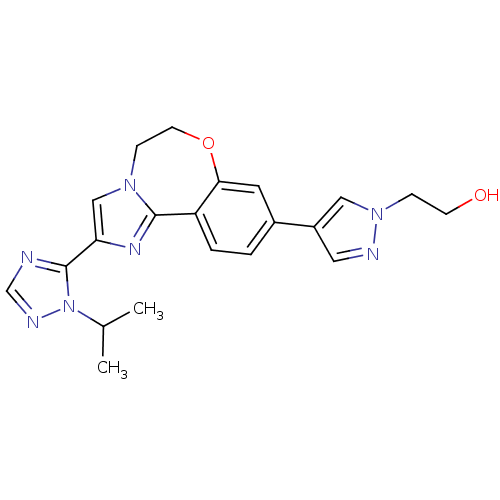
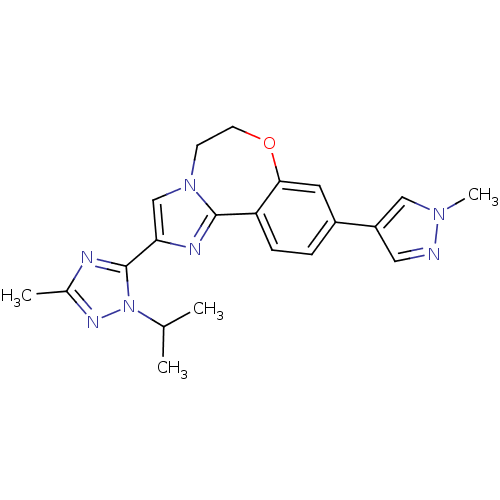
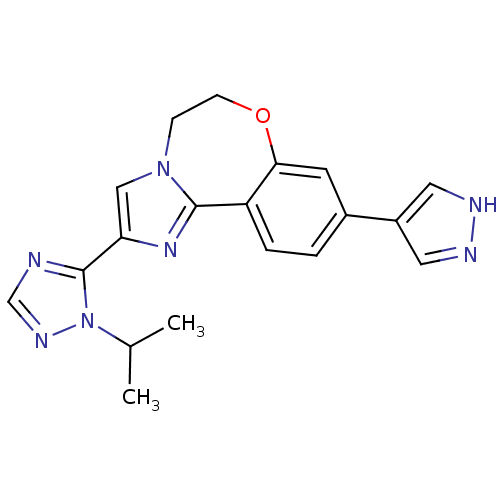
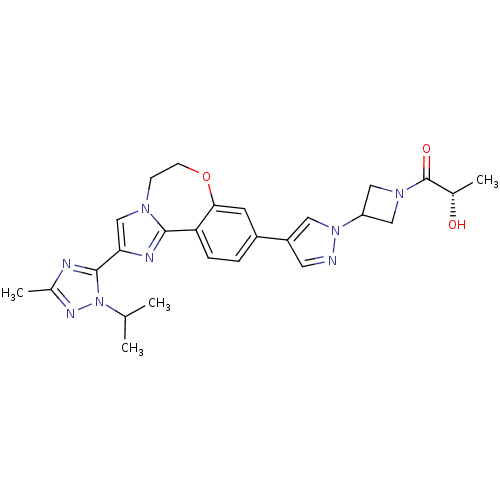
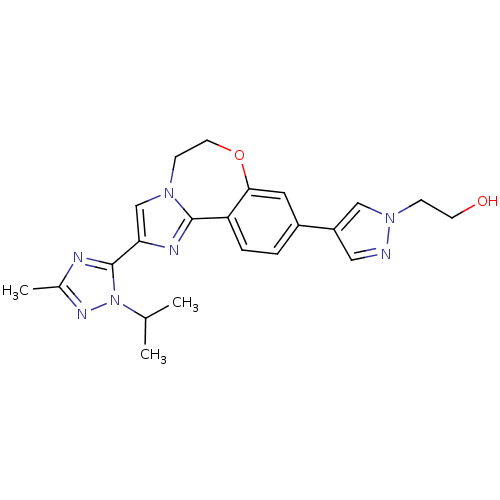
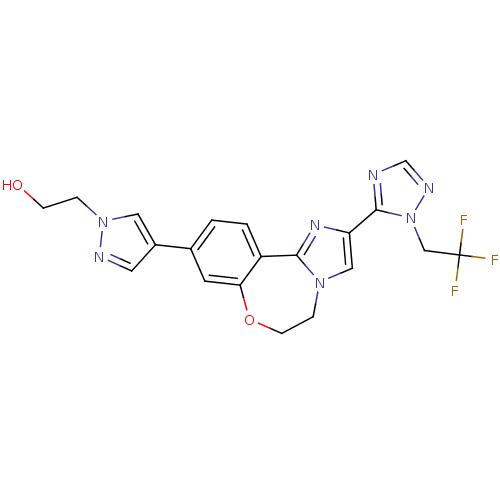
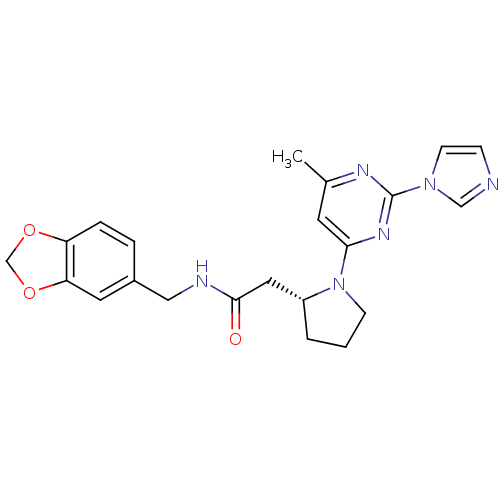
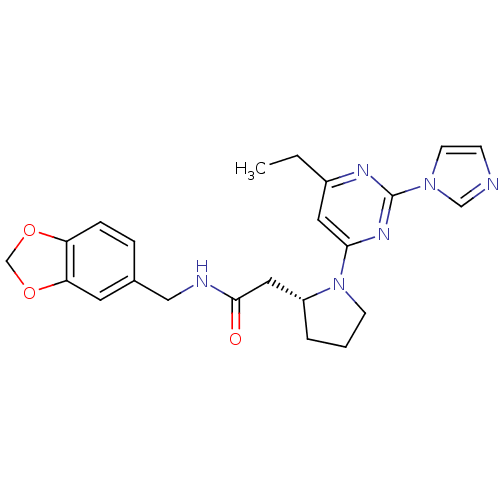
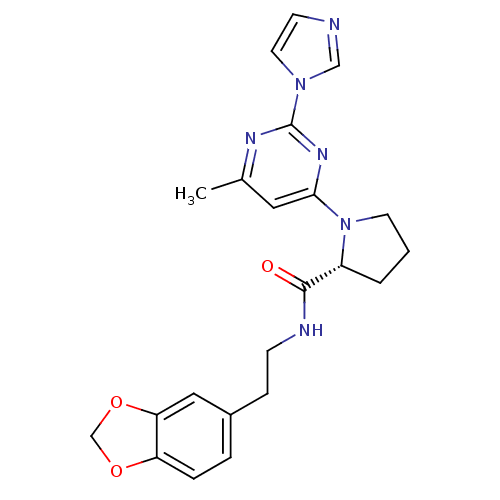
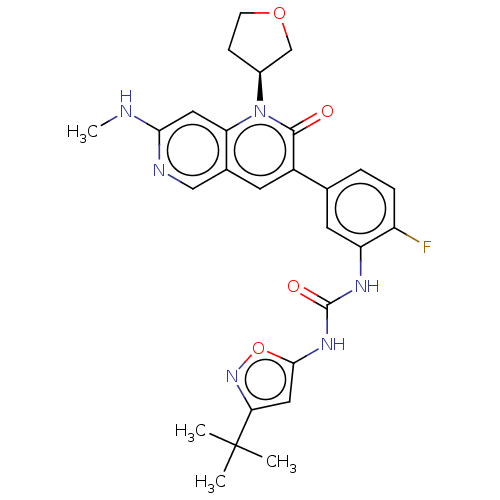
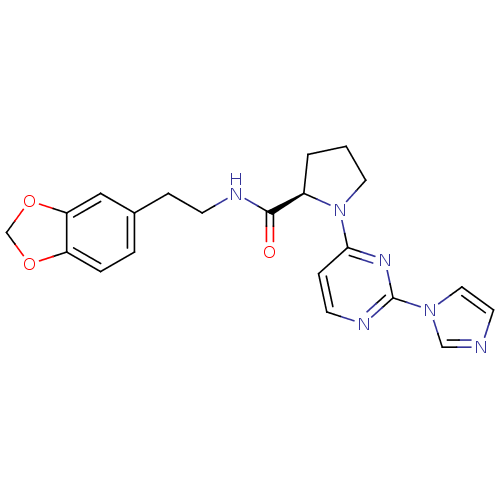
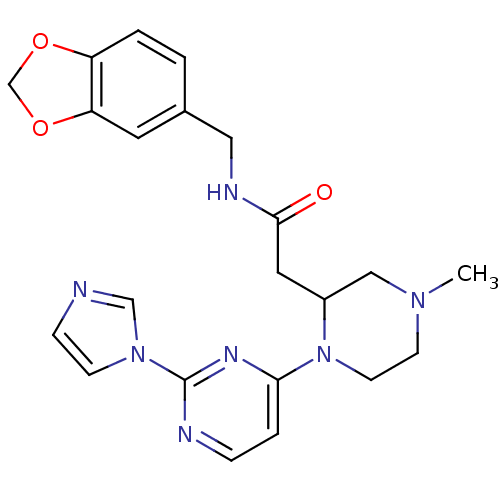
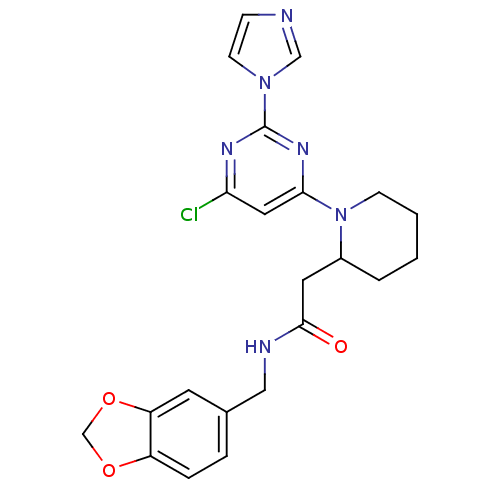
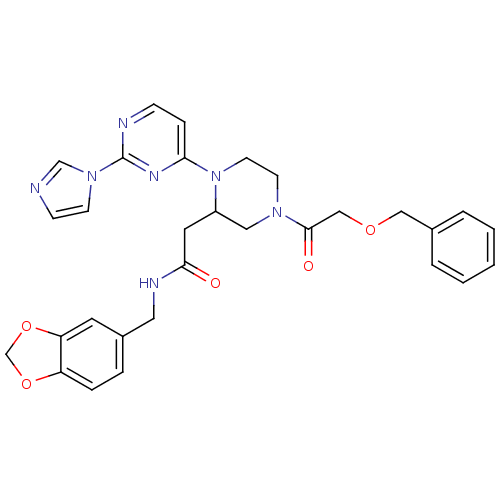
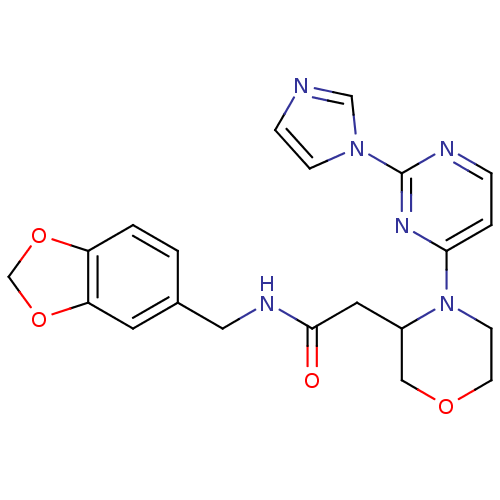
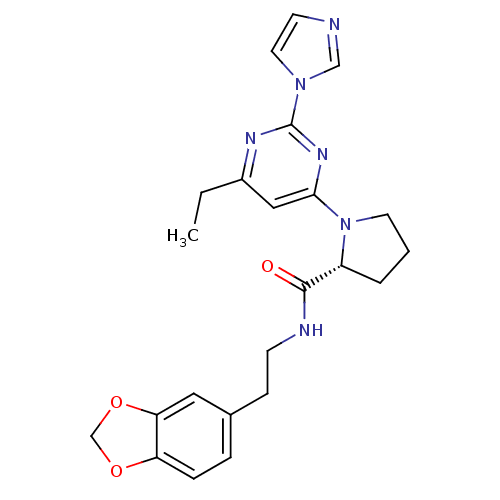
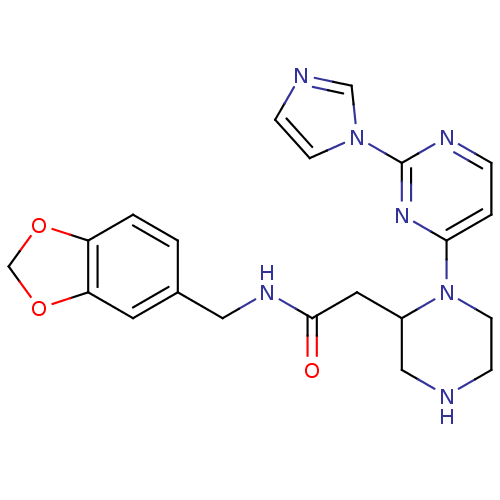
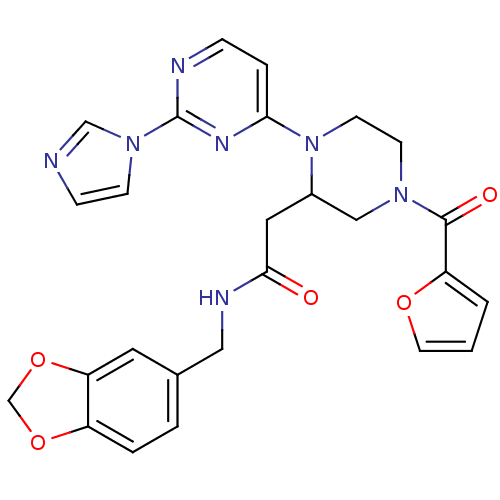
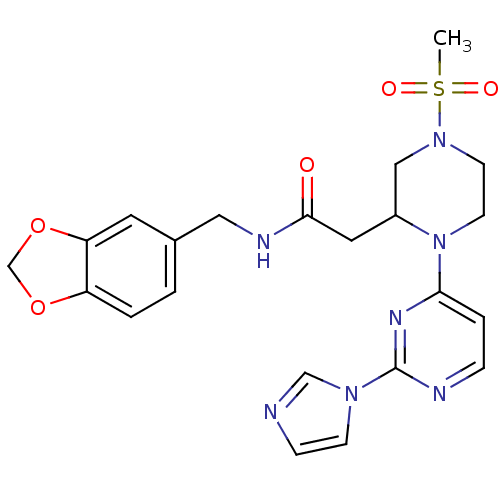
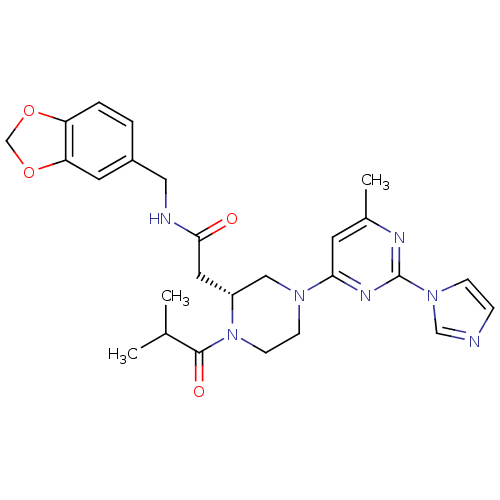
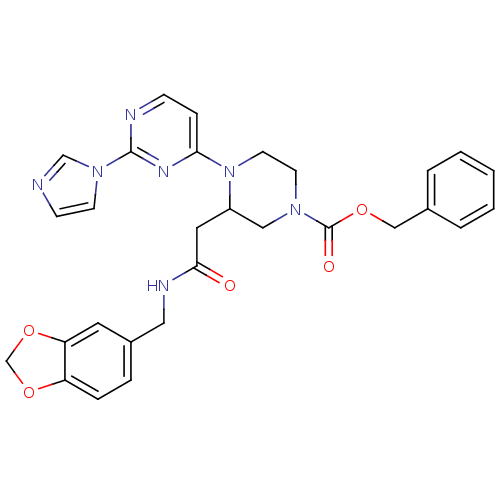
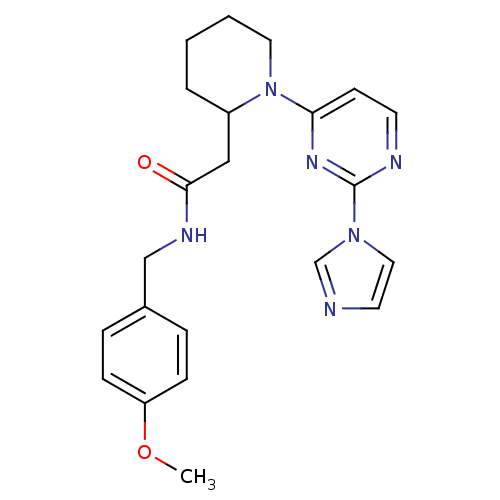
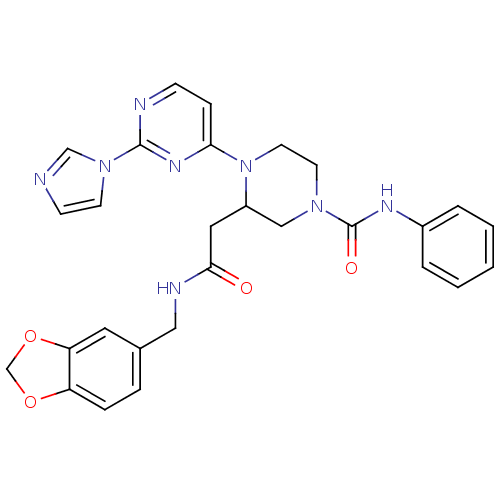
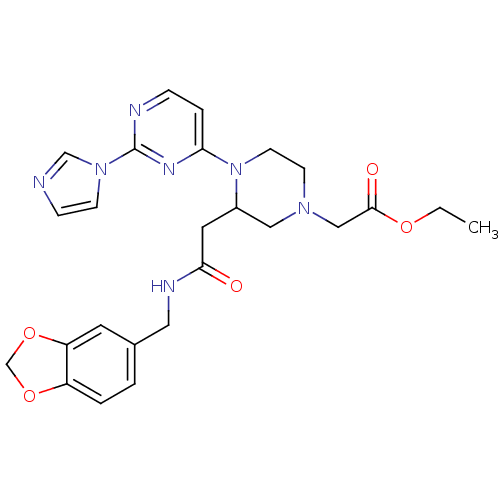
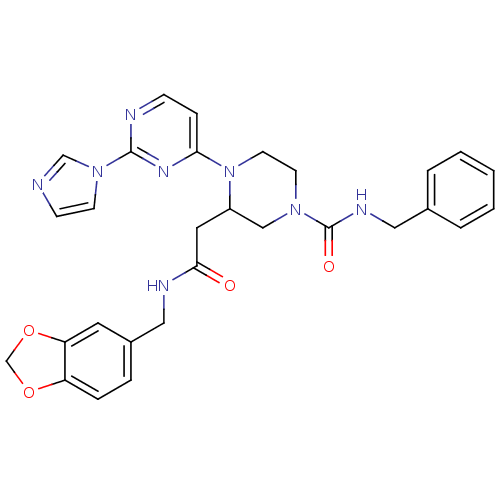
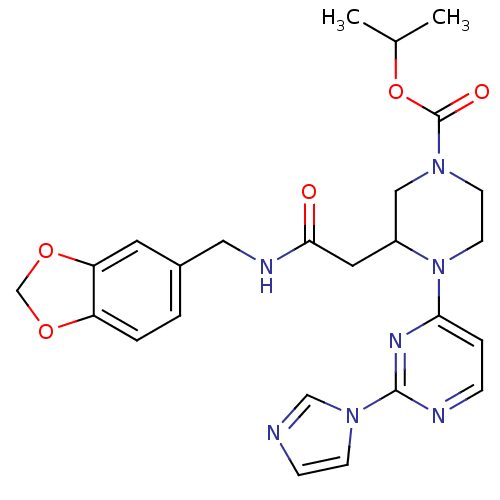
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.110nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
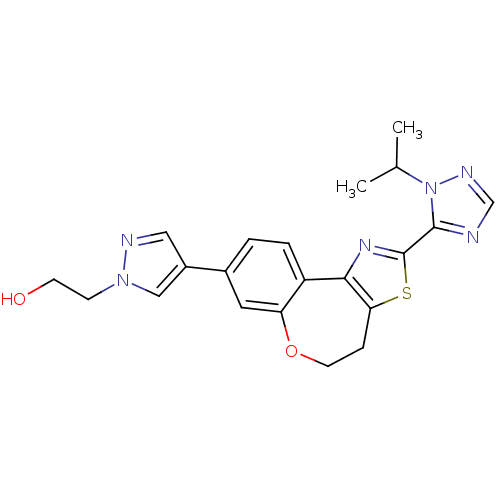
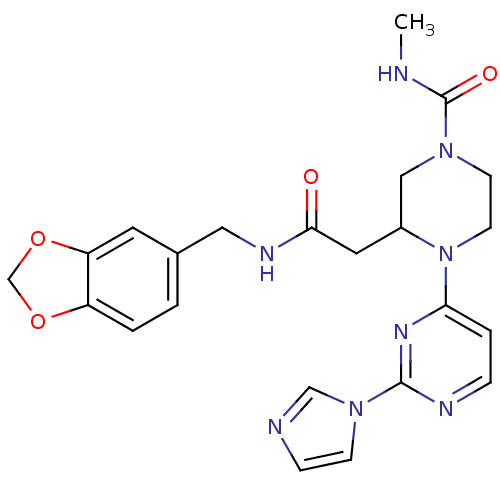
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.120nMAssay Description:Inhibition of PI3Kdelta (unknown origin)More data for this Ligand-Target Pair
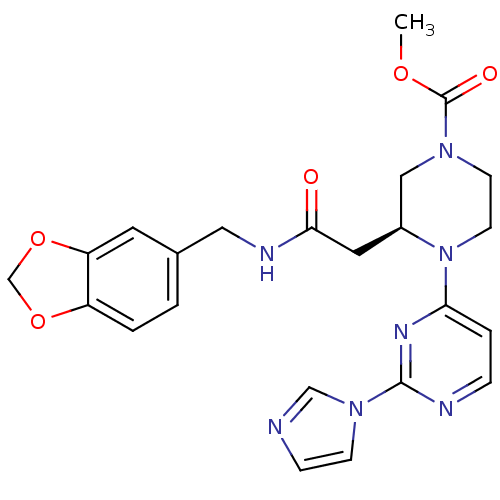
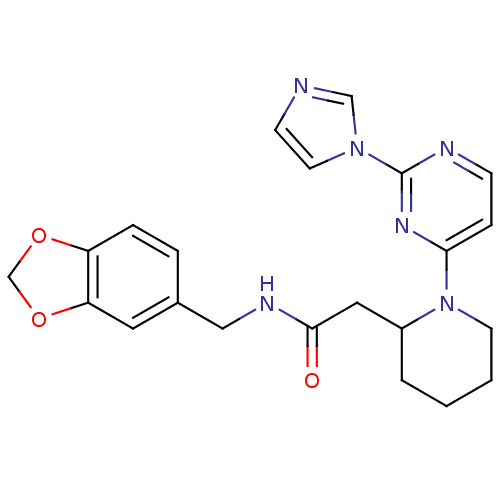
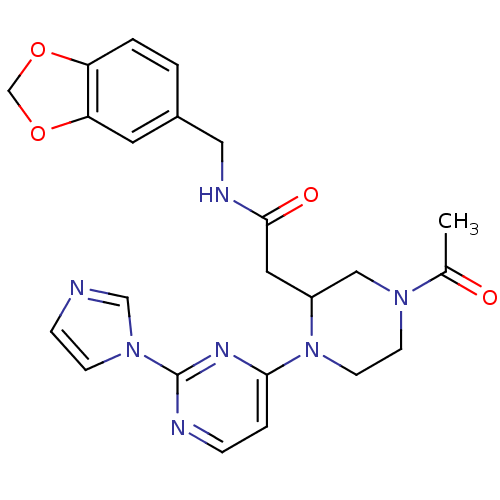
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.140nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
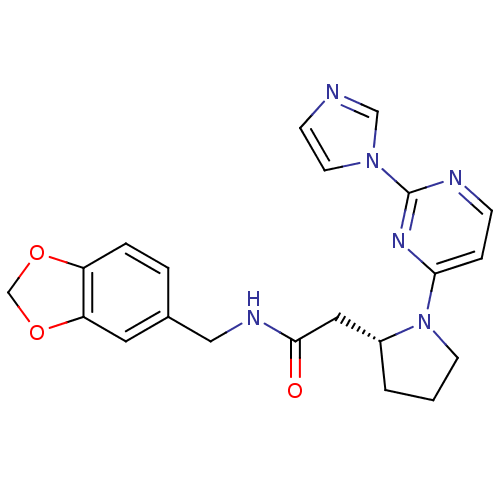
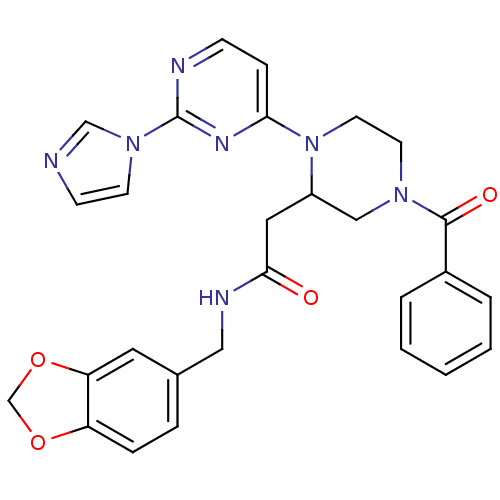
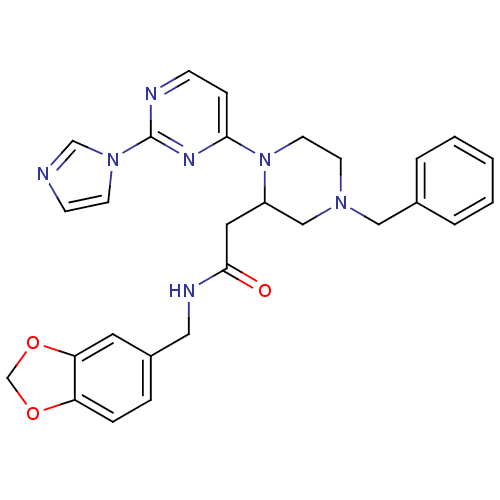
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.170nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
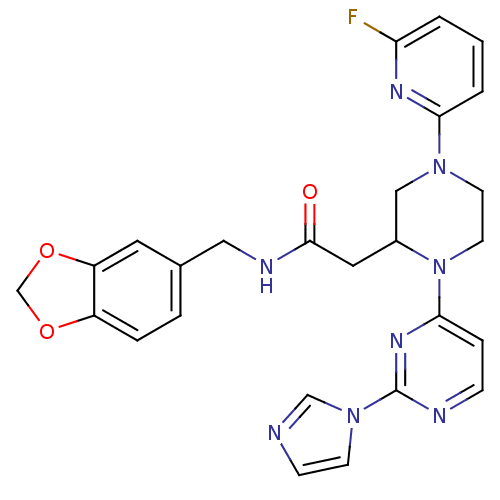
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.290nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.290nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.300nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.310nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.340nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.400nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.970nMAssay Description:Inhibition of PI3Kgamma (unknown origin)More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: <1nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 2.80nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 9.10nMAssay Description:Inhibition of PI3Kbeta (unknown origin)More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 21nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.20E+3nMAssay Description:Inhibition of mTOR (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.120nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.130nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.240nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.280nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.280nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.290nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.380nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.460nMAssay Description:Inhibition of KDR (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.480nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.480nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.490nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: <0.5nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.520nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.550nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.580nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.670nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 0.710nMAssay Description:Inhibition of PI3Kalpha in human MCF7-neo/Her2 cells assessed as reduction of AKT phosphorylation at S473More data for this Ligand-Target Pair
Affinity DataIC50: 0.75nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.870nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.930nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 0.930nMAssay Description:Inhibition of PI3Kalpha in human MCF7-neo/Her2 cells assessed as reduction of AKT phosphorylation at S473More data for this Ligand-Target Pair
Affinity DataIC50: 0.960nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of recombinant BRAF V600E mutant (unknown origin) assessed as ADP formation measured for 5 hrs by pyruvate kinase/lactate dehydrogenase co...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)