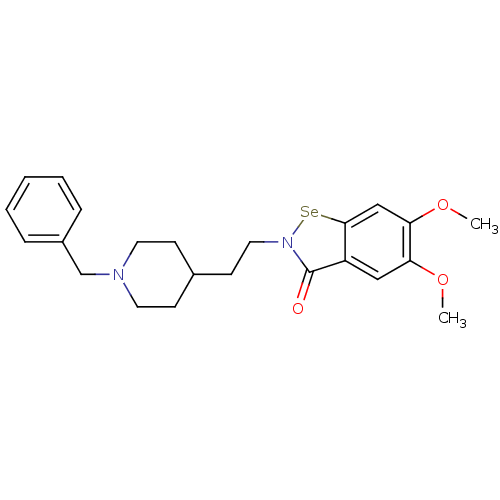
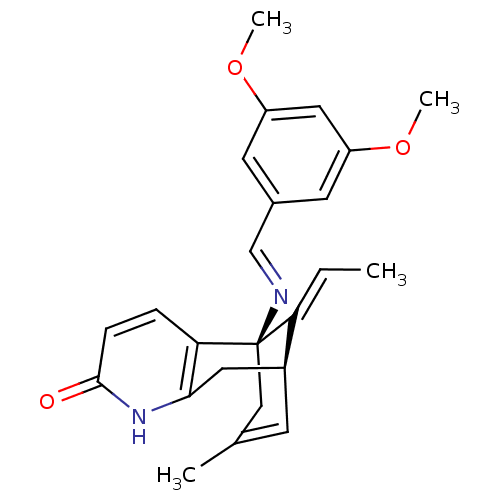
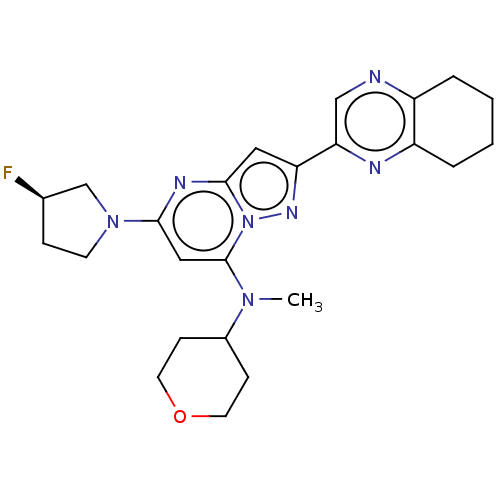
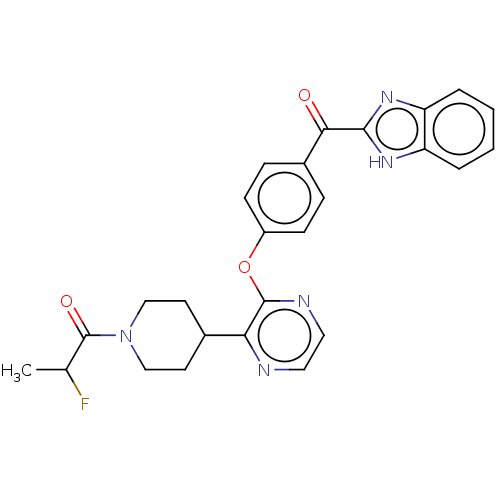
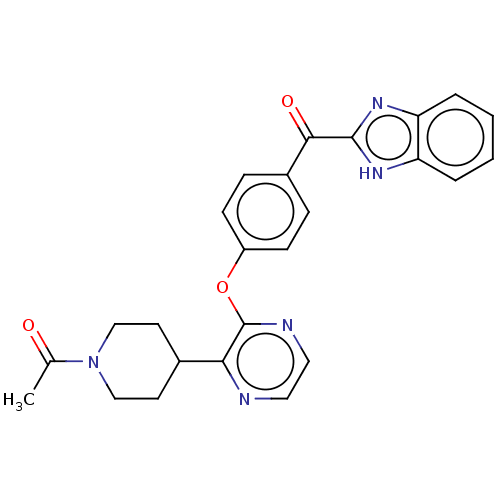
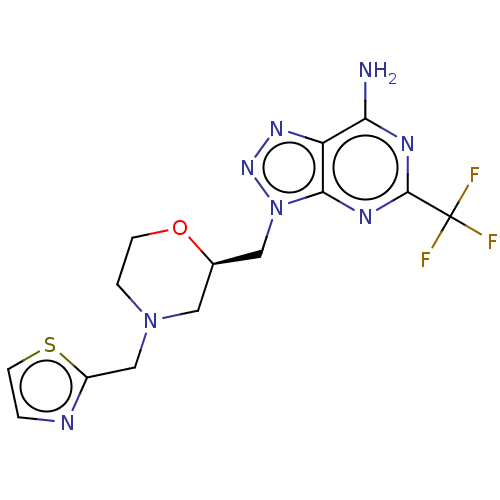
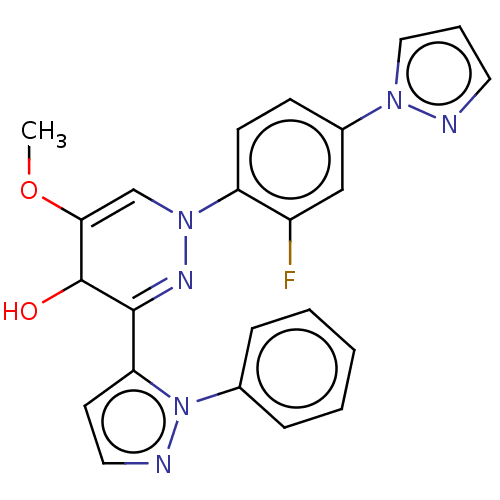
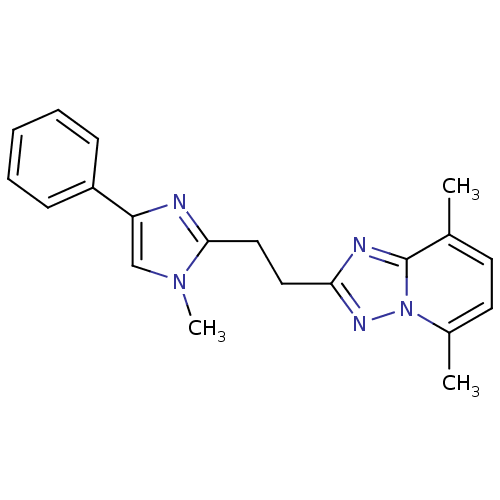
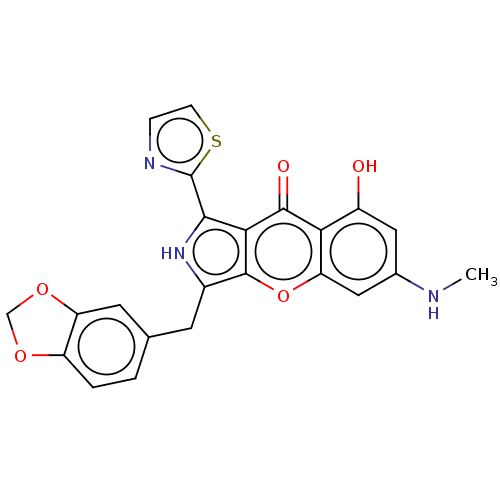
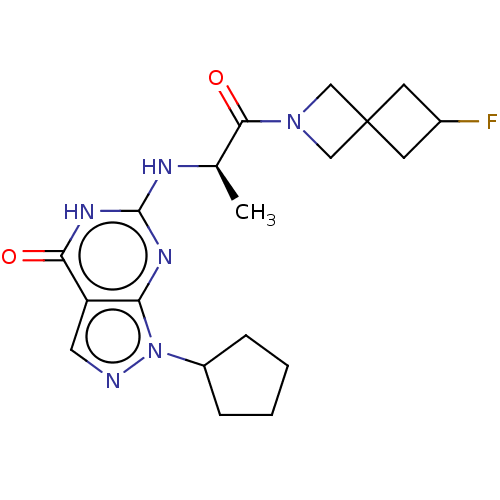
TargetTyrosine-protein kinase receptor(Homo sapiens (Human))
Chia Tai Tianqing Pharmaceutical Group
US Patent
Chia Tai Tianqing Pharmaceutical Group
US Patent
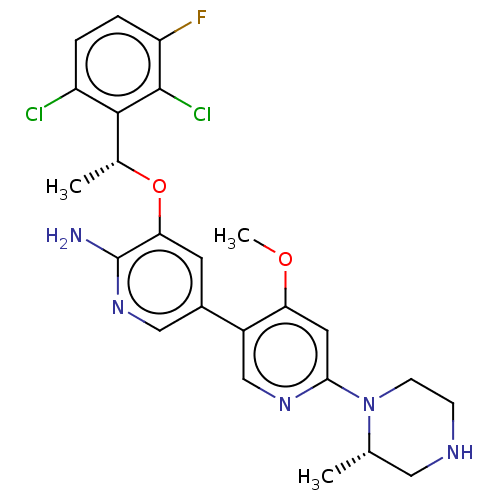
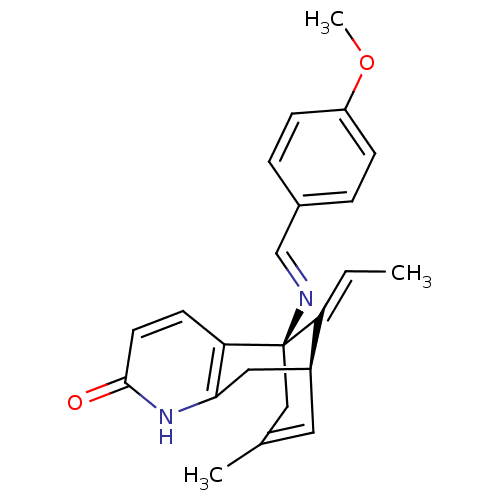
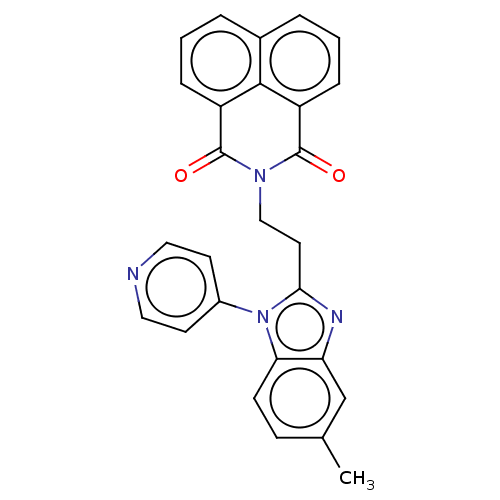
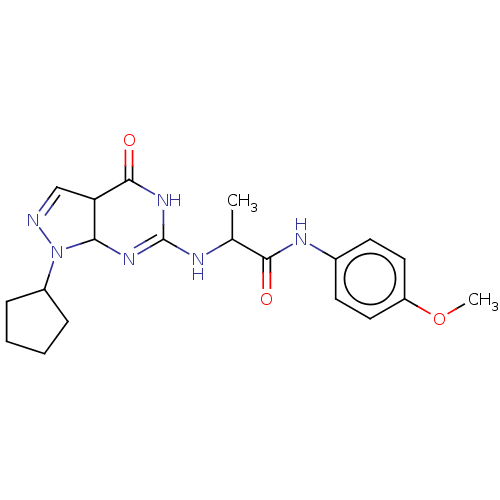
TargetTyrosine-protein kinase receptor(Homo sapiens (Human))
Chia Tai Tianqing Pharmaceutical Group
US Patent
Chia Tai Tianqing Pharmaceutical Group
US Patent
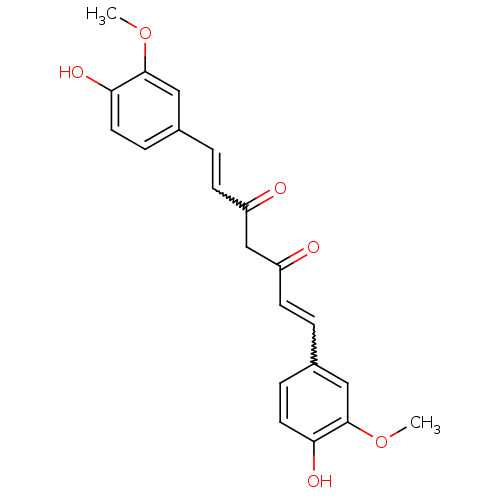
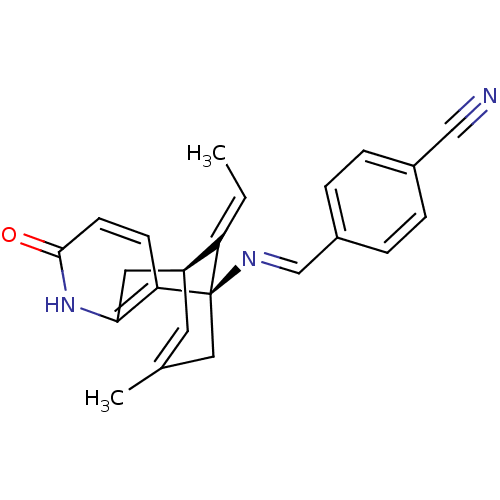
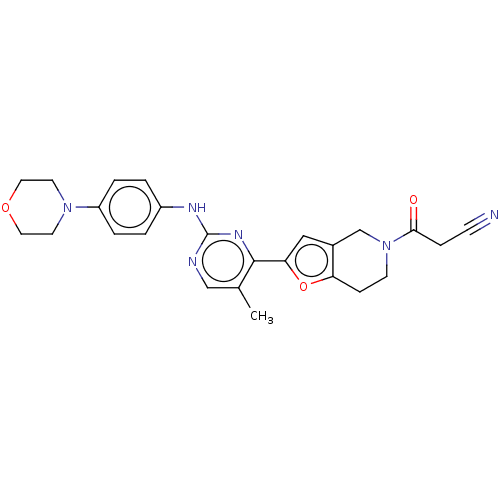
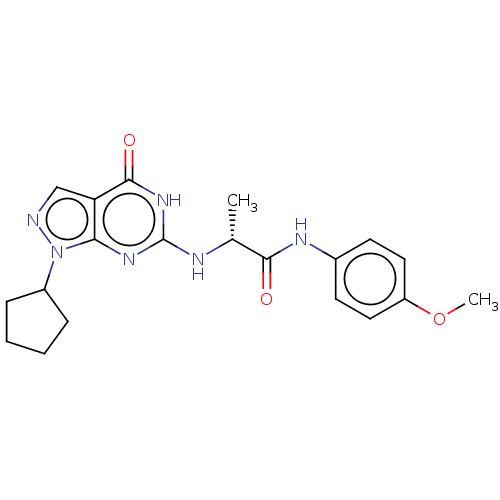
TargetTyrosine-protein kinase receptor(Homo sapiens (Human))
Chia Tai Tianqing Pharmaceutical Group
US Patent
Chia Tai Tianqing Pharmaceutical Group
US Patent
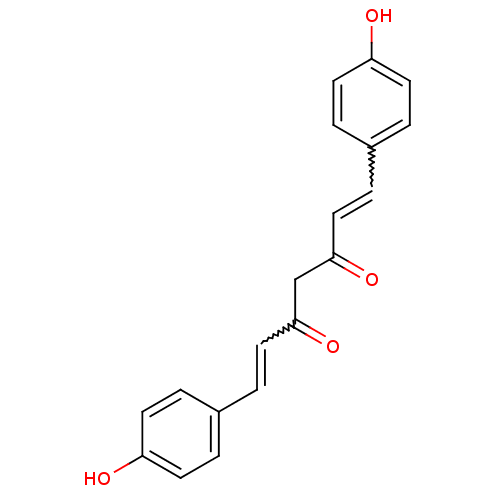
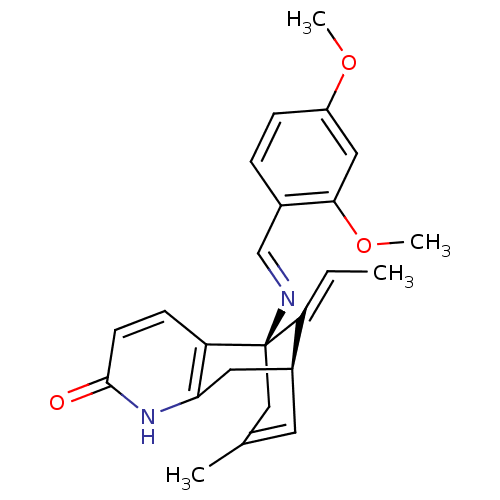
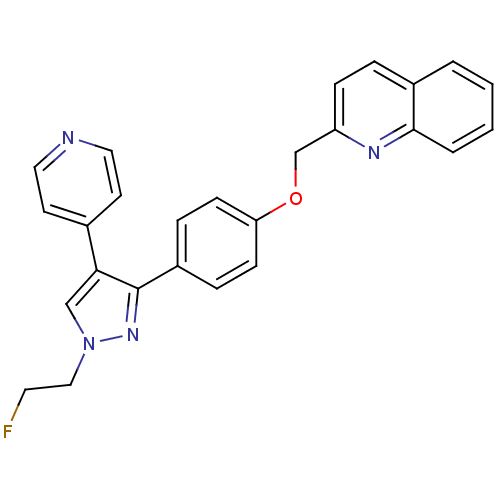
TargetTyrosine-protein kinase receptor(Homo sapiens (Human))
Chia Tai Tianqing Pharmaceutical Group
US Patent
Chia Tai Tianqing Pharmaceutical Group
US Patent
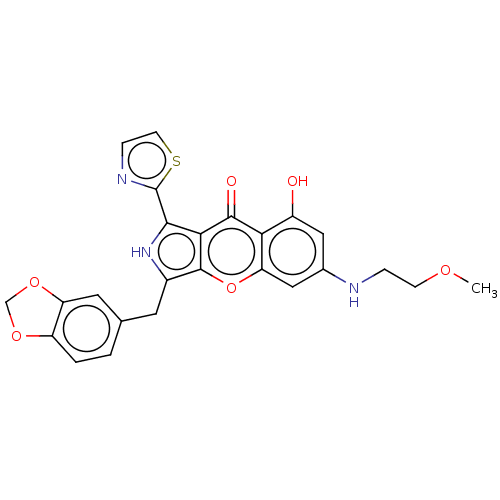
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataKi: 7nMAssay Description:Binding affinity to PDE9A2 (unknown origin)More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataKi: 17nMAssay Description:Competitive inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addition by ...More data for this Ligand-Target Pair
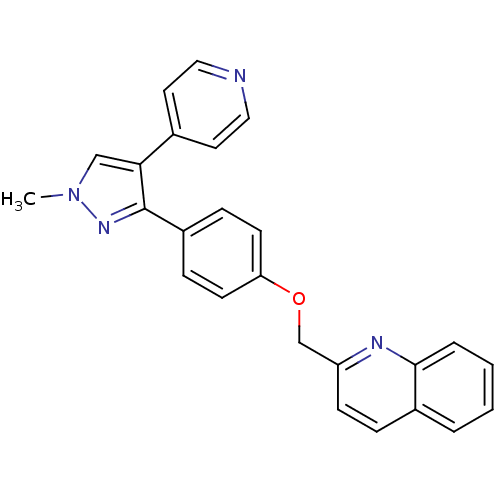
TargetALK tyrosine kinase receptor [L1196M](Homo sapiens (Human))
Chia Tai Tianqing Pharmaceutical Group
US Patent
Chia Tai Tianqing Pharmaceutical Group
US Patent
TargetALK tyrosine kinase receptor [L1196M](Homo sapiens (Human))
Chia Tai Tianqing Pharmaceutical Group
US Patent
Chia Tai Tianqing Pharmaceutical Group
US Patent
TargetALK tyrosine kinase receptor [L1196M](Homo sapiens (Human))
Chia Tai Tianqing Pharmaceutical Group
US Patent
Chia Tai Tianqing Pharmaceutical Group
US Patent
TargetALK tyrosine kinase receptor [G1269S](Homo sapiens (Human))
Chia Tai Tianqing Pharmaceutical Group
US Patent
Chia Tai Tianqing Pharmaceutical Group
US Patent
TargetALK tyrosine kinase receptor [G1269S](Homo sapiens (Human))
Chia Tai Tianqing Pharmaceutical Group
US Patent
Chia Tai Tianqing Pharmaceutical Group
US Patent
TargetALK tyrosine kinase receptor [G1269S](Homo sapiens (Human))
Chia Tai Tianqing Pharmaceutical Group
US Patent
Chia Tai Tianqing Pharmaceutical Group
US Patent
Affinity DataKi: 1.03E+4nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
Affinity DataKi: 1.82E+4nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
Affinity DataKi: 2.44E+4nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
Affinity DataKi: 8.78E+4nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
Affinity DataKi: 3.35E+5nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
Affinity DataKi: 3.83E+5nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
Affinity DataKi: 8.43E+5nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Tetronarce californica (Pacific electric ray) (Tor...)
The Chinese Academy Of Sciences
Curated by ChEMBL
The Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 0.0200nMAssay Description:Inhibition of Torpedo california AChE by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Tetronarce californica (Pacific electric ray) (Tor...)
The Chinese Academy Of Sciences
Curated by ChEMBL
The Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 0.0246nMAssay Description:Inhibition of Torpedo california AChE by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Tetronarce californica (Pacific electric ray) (Tor...)
The Chinese Academy Of Sciences
Curated by ChEMBL
The Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 0.0412nMAssay Description:Inhibition of Torpedo california AChE by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Tetronarce californica (Pacific electric ray) (Tor...)
The Chinese Academy Of Sciences
Curated by ChEMBL
The Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 0.0446nMAssay Description:Inhibition of Torpedo california AChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0600nMAssay Description:Antagonist activity at CCK2 receptor in immature rat stomach assessed as pentagastrin-induced acid secretionMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Chia Tai Tianqing Pharmaceutical Group
US Patent
Chia Tai Tianqing Pharmaceutical Group
US Patent
Affinity DataIC50: 0.100nMAssay Description:EGFR, EGFR (T790M, L858R), HER2 kinase were expressed and purified through an insect cell expression system by the Department of Biology, Centaurus B...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Tetronarce californica (Pacific electric ray) (Tor...)
The Chinese Academy Of Sciences
Curated by ChEMBL
The Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 0.121nMAssay Description:Inhibition of Torpedo california AChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.130nMAssay Description:Antagonist activity at CCK2 receptor in immature rat stomach assessed as pentagastrin-induced acid secretionMore data for this Ligand-Target Pair
Affinity DataIC50: 0.180nMAssay Description:Antagonist activity at CCK2 receptor in immature rat stomach assessed as pentagastrin-induced acid secretionMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Chia Tai Tianqing Pharmaceutical Group
US Patent
Chia Tai Tianqing Pharmaceutical Group
US Patent
Affinity DataIC50: 0.200nMAssay Description:EGFR, EGFR (T790M, L858R), HER2 kinase were expressed and purified through an insect cell expression system by the Department of Biology, Centaurus B...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Chia Tai Tianqing Pharmaceutical Group
US Patent
Chia Tai Tianqing Pharmaceutical Group
US Patent
Affinity DataIC50: 0.200nMAssay Description:EGFR, EGFR (T790M, L858R), HER2 kinase were expressed and purified through an insect cell expression system by the Department of Biology, Centaurus B...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Chia Tai Tianqing Pharmaceutical Group
US Patent
Chia Tai Tianqing Pharmaceutical Group
US Patent
Affinity DataIC50: 0.200nMAssay Description:EGFR, EGFR (T790M, L858R), HER2 kinase were expressed and purified through an insect cell expression system by the Department of Biology, Centaurus B...More data for this Ligand-Target Pair
TargetHigh affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B(Homo sapiens (Human))TBA
Affinity DataIC50: 0.300nMAssay Description:Antagonist activity at AT1 receptor assessed inhibition of angiotensin-2-induced contraction of rabbit thoracic aortic ringsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibition of human PDE10A2 catalytic domain (446 to 789 residues) expressed in Escherichia coli BL21 cells using [3H]-cGMP as substrate after 15 min...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Chia Tai Tianqing Pharmaceutical Group
US Patent
Chia Tai Tianqing Pharmaceutical Group
US Patent
Affinity DataIC50: 0.300nMAssay Description:EGFR, EGFR (T790M, L858R), HER2 kinase were expressed and purified through an insect cell expression system by the Department of Biology, Centaurus B...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Chia Tai Tianqing Pharmaceutical Group
US Patent
Chia Tai Tianqing Pharmaceutical Group
US Patent
Affinity DataIC50: 0.300nMAssay Description:EGFR, EGFR (T790M, L858R), HER2 kinase were expressed and purified through an insect cell expression system by the Department of Biology, Centaurus B...More data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 0.320nMAssay Description:Inhibition of human PDE5A1 catalytic domain (535 to 860 residues) expressed in Escherichia coli BL21 using 3H-cGMP as substrate after 15 mins by liqu...More data for this Ligand-Target Pair
Affinity DataIC50: 0.370nMAssay Description:Inhibition of human PDE10A2 catalytic domain (446 to 789 residues) expressed in Escherichia coli BL21 cells using [3H]-cGMP as substrate after 15 min...More data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 0.390nMAssay Description:Inhibition of human PDE5A1 catalytic domain (535 to 860 residues) expressed in Escherichia coli BL21 using 3H-cGMP as substrate after 15 mins by liqu...More data for this Ligand-Target Pair
Affinity DataIC50: 0.390nMAssay Description:Inhibition of P-glycoprotein expressed in MDCK-MDR1 cells by calcein AM assayMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Chia Tai Tianqing Pharmaceutical Group
US Patent
Chia Tai Tianqing Pharmaceutical Group
US Patent
Affinity DataIC50: 0.400nMAssay Description:EGFR, EGFR (T790M, L858R), HER2 kinase were expressed and purified through an insect cell expression system by the Department of Biology, Centaurus B...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D(Homo sapiens (Human))
National-Local Joint Engineering Laboratory Of Druggability And New Drugs Evaluation
Curated by ChEMBL
National-Local Joint Engineering Laboratory Of Druggability And New Drugs Evaluation
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of PDE4D (unknown origin)More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Chia Tai Tianqing Pharmaceutical Group
US Patent
Chia Tai Tianqing Pharmaceutical Group
US Patent
Affinity DataIC50: 0.400nMAssay Description:EGFR, EGFR (T790M, L858R), HER2 kinase were expressed and purified through an insect cell expression system by the Department of Biology, Centaurus B...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D(Homo sapiens (Human))
National-Local Joint Engineering Laboratory Of Druggability And New Drugs Evaluation
Curated by ChEMBL
National-Local Joint Engineering Laboratory Of Druggability And New Drugs Evaluation
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of PDE4D2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.440nMAssay Description:Inhibition of human PDE10A2 catalytic domain (446 to 789 residues) expressed in Escherichia coli BL21 cells using [3H]-cGMP as substrate after 15 min...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase JAK2(Homo sapiens (Human))
Central China Normal University
Curated by ChEMBL
Central China Normal University
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of JAK2 (unknown origin) using TK-substrate-biotin as substrate preincubated for 5 mins followed by substrate addition and measured by 30 ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Antagonist activity at CCK2 receptor in immature rat stomach assessed as pentagastrin-induced acid secretionMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 0.520nMAssay Description:Inhibition of human PDE5A1 catalytic domain (535 to 860 residues) expressed in Escherichia coli BL21 using 3H-cGMP as substrate after 15 mins by liqu...More data for this Ligand-Target Pair
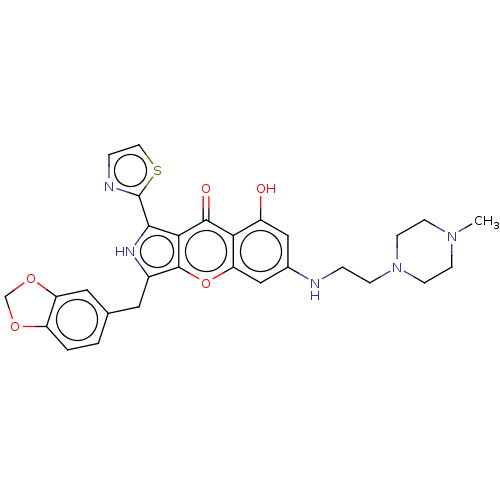
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Chia Tai Tianqing Pharmaceutical Group
US Patent
Chia Tai Tianqing Pharmaceutical Group
US Patent
Affinity DataIC50: 0.600nMAssay Description:EGFR, EGFR (T790M, L858R), HER2 kinase were expressed and purified through an insect cell expression system by the Department of Biology, Centaurus B...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of PDE9A (unknown origin)More data for this Ligand-Target Pair


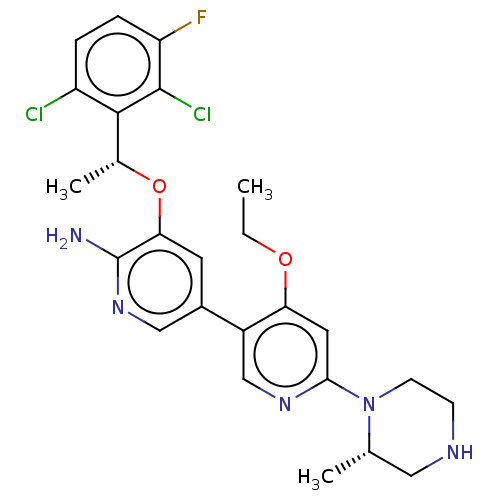
 3D Structure (crystal)
3D Structure (crystal)