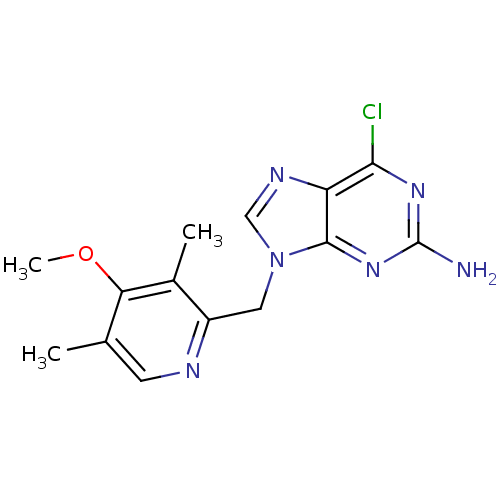
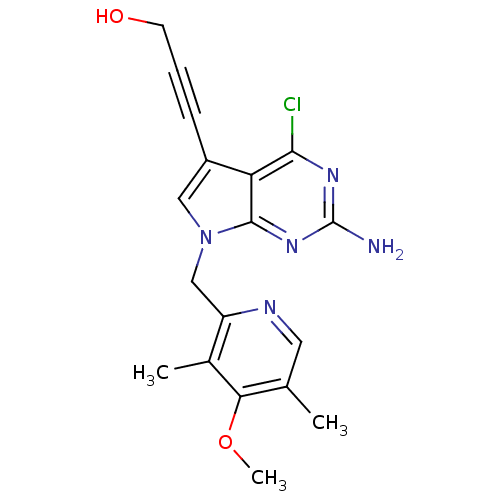
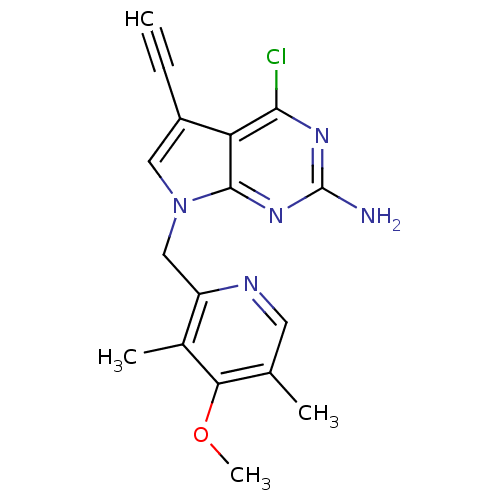
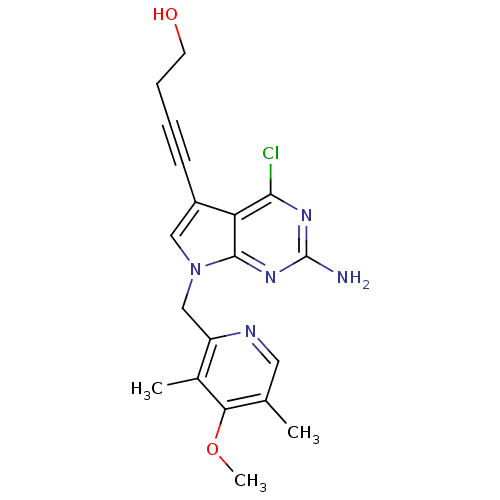
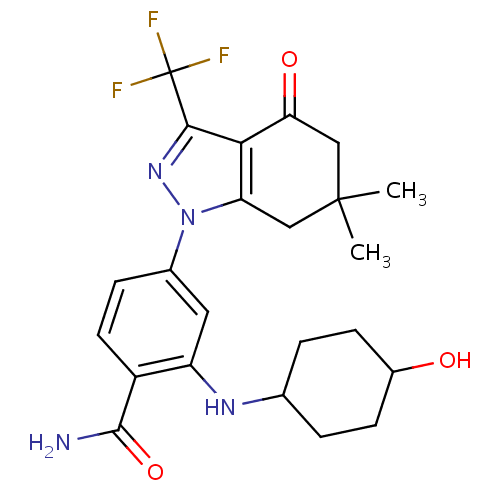
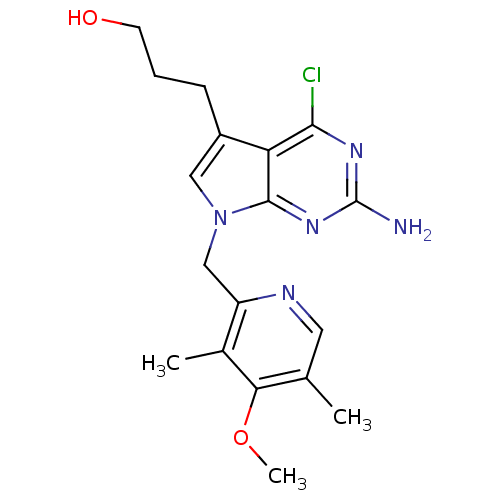
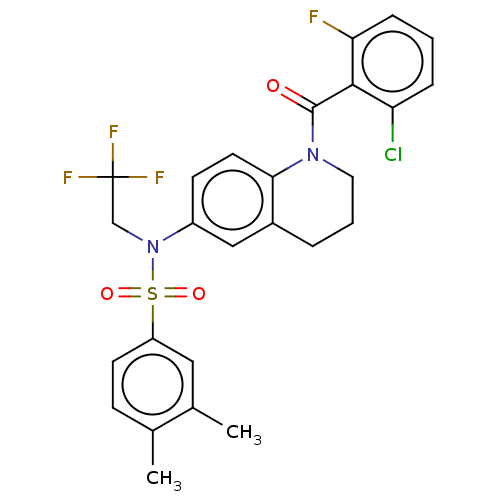
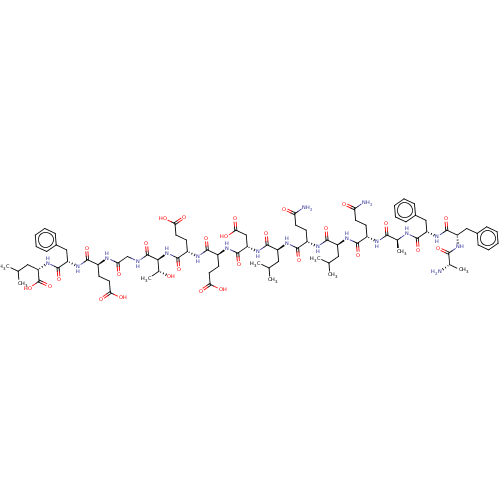
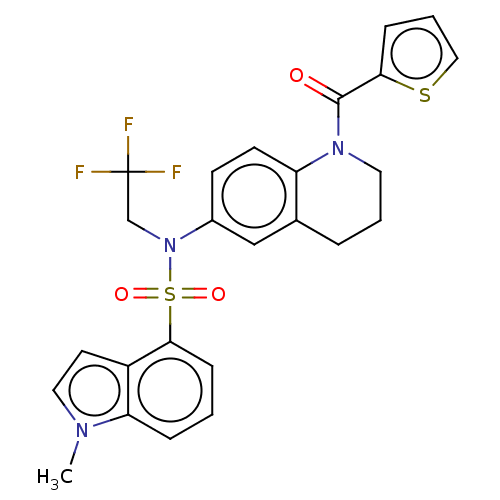
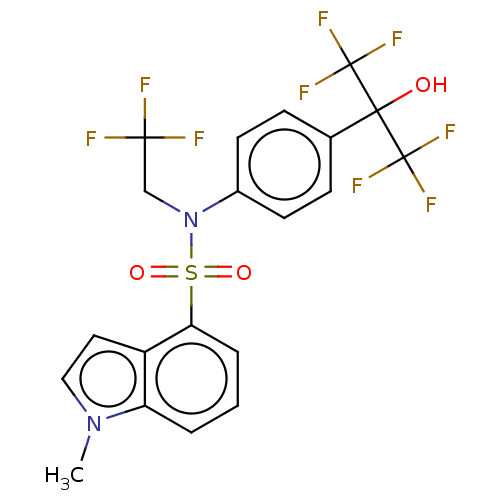
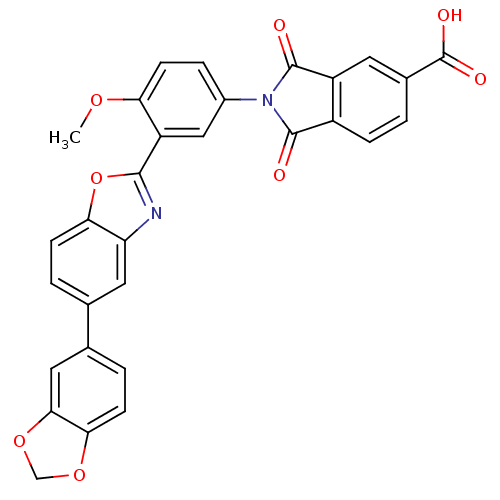
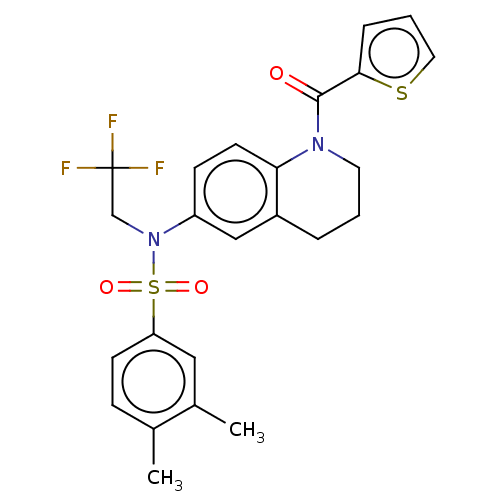
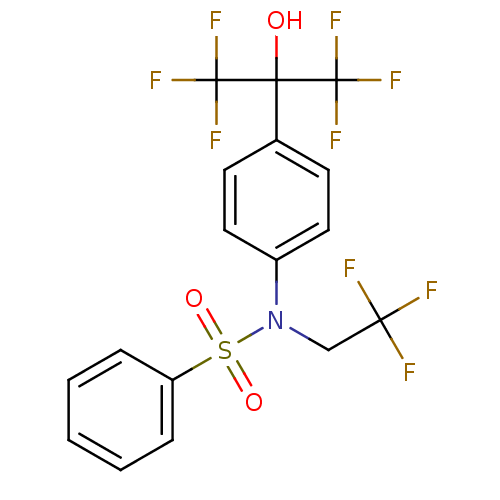
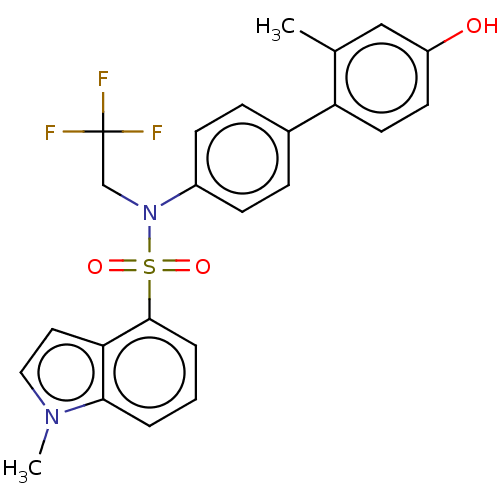
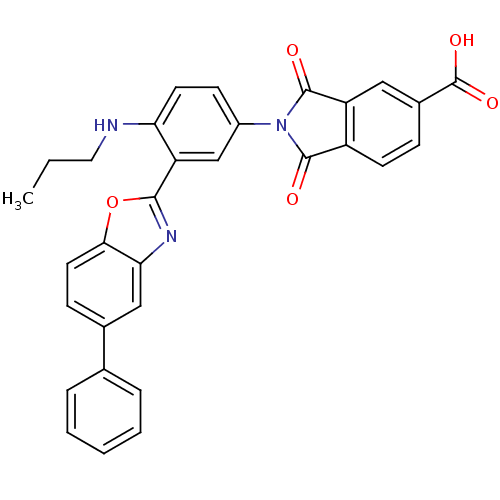
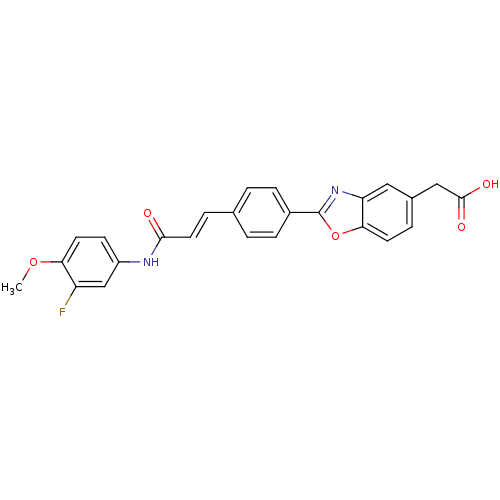
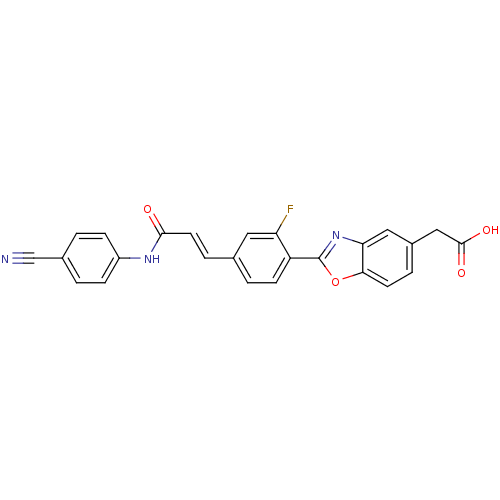
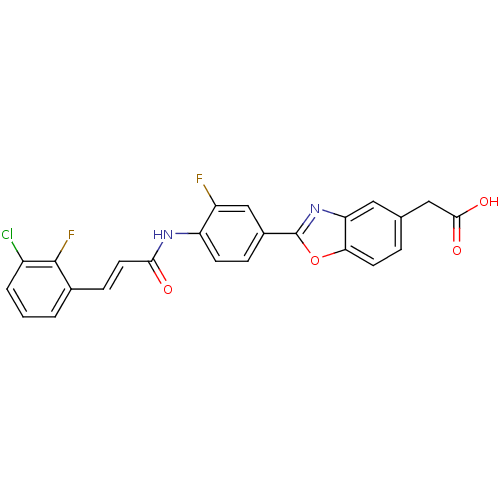
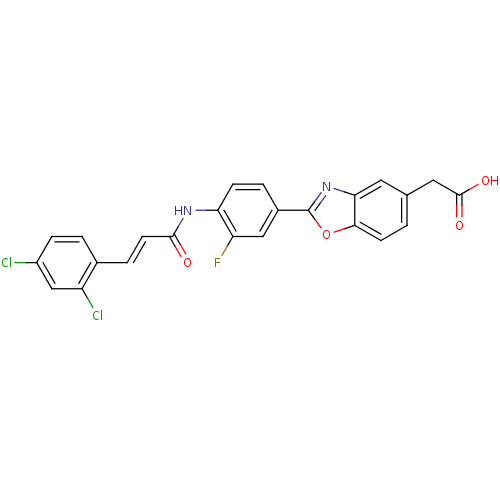
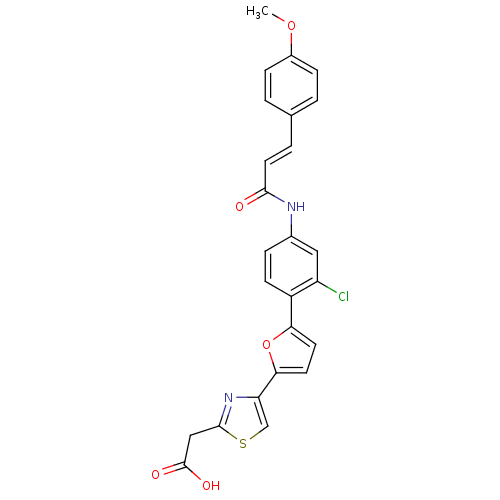
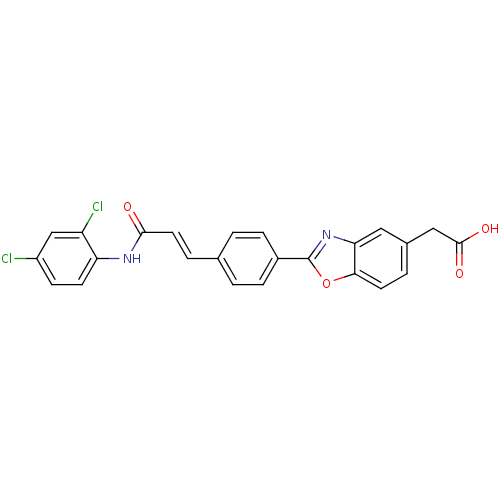
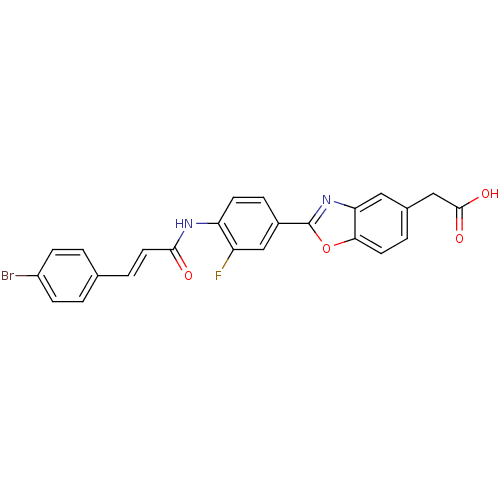
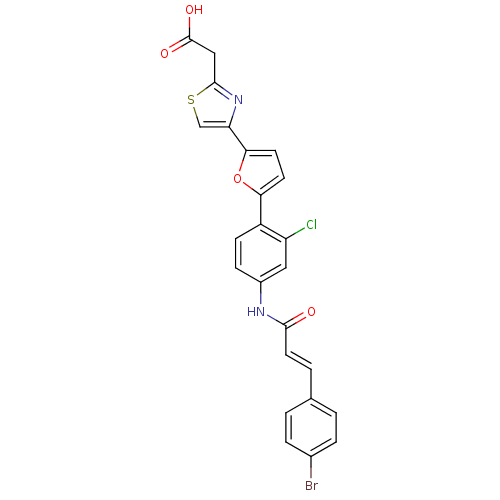
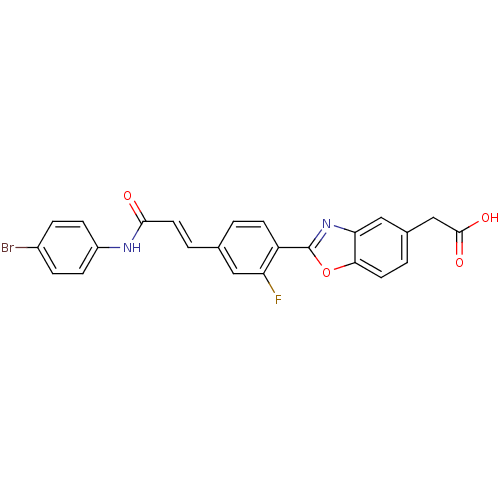
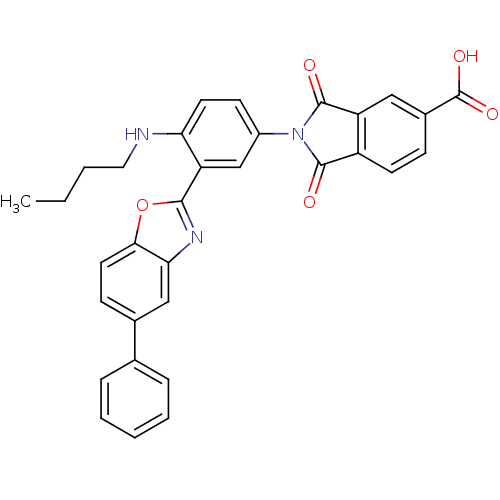
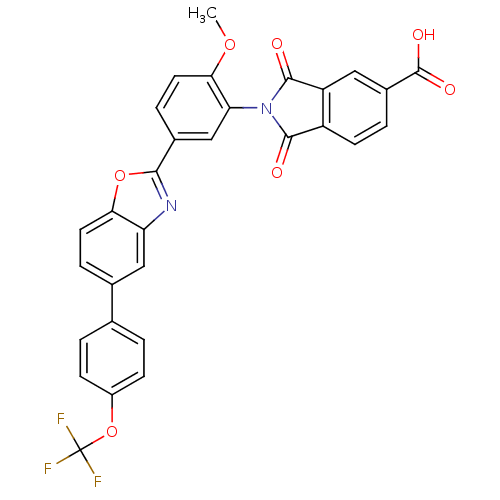
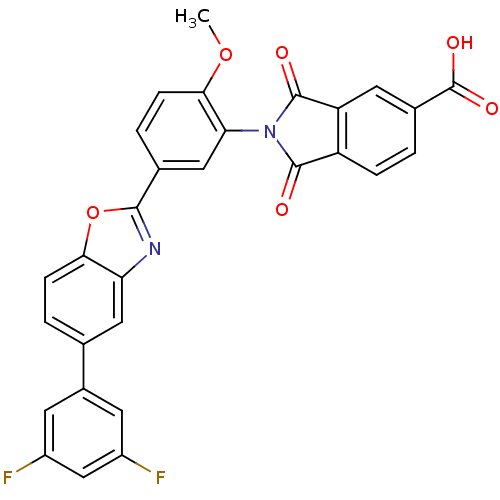
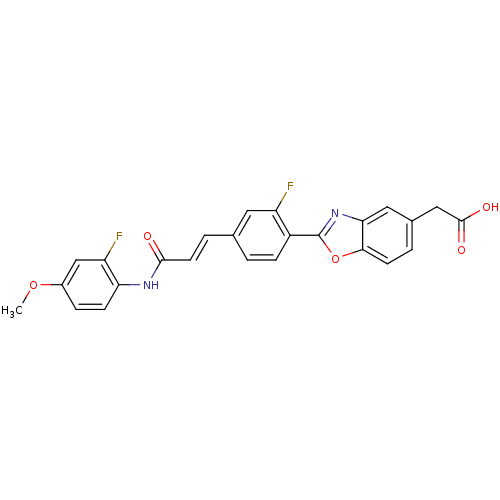
Affinity DataKi: 0.200nMAssay Description:Binding affinity at recombinant Hsp90alpha incubated for 16 hrs by fluorescence polarization competition assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.70nMAssay Description:Binding affinity at recombinant Hsp90alpha incubated for 16 hrs by fluorescence polarization competition assayMore data for this Ligand-Target Pair
Affinity DataKi: 61nMAssay Description:Binding affinity at Grp94 incubated for 16 hrs by fluorescence polarization competition assayMore data for this Ligand-Target Pair
Affinity DataKi: 255nMAssay Description:Binding affinity at TRAP1 incubated for 16 hrs by fluorescence polarization competition assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Inhibition of Hsp90alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of Hsp90alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Inhibition of Hsp90alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of Hsp90alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of Hsp90alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 3.90nMAssay Description:Inhibition of Hsp90alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 5.10nMAssay Description:Inhibition of Hsp90alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of Hsp90alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inverse agonist activity at recombinant human GST-tagged ROR-gamma receptor ligand binding domain assessed as inhibition of receptor and co-activator...More data for this Ligand-Target Pair
Affinity DataIC50: 33nMAssay Description:Displacement of MR121-Nrf2 from human N-terminal His-tagged Kelch-DC domain of Keap1 (321 to 609) expressed in Escherichia coli BL21 (DE3) after 40 m...More data for this Ligand-Target Pair
Affinity DataIC50: 56nMAssay Description:Inverse agonist activity at recombinant human GST-tagged ROR-gamma receptor ligand binding domain assessed as inhibition of receptor and co-activator...More data for this Ligand-Target Pair
Affinity DataIC50: 72nMAssay Description:Inverse agonist activity at recombinant human GST-tagged ROR-gamma receptor ligand binding domain assessed as inhibition of receptor and co-activator...More data for this Ligand-Target Pair
Affinity DataIC50: 72nMAssay Description:Inverse agonist activity at recombinant human GST-tagged ROR-gamma receptor ligand binding domain assessed as inhibition of receptor and co-activator...More data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:In vitro inhibitory concentration against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:In vitro inhibitory concentration against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:In vivo inhibitory activity against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:In vitro inhibitory concentration against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inverse agonist activity at recombinant human GST-tagged ROR-gamma receptor ligand binding domain assessed as inhibition of receptor and co-activator...More data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:In vivo inhibitory activity against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:In vitro inhibitory concentration against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 204nMAssay Description:Inverse agonist activity at recombinant human GST-tagged ROR-gamma receptor ligand binding domain assessed as inhibition of receptor and co-activator...More data for this Ligand-Target Pair
Affinity DataIC50: 210nMAssay Description:Inverse agonist activity at recombinant human GST-tagged ROR-gamma receptor ligand binding domain assessed as inhibition of receptor and co-activator...More data for this Ligand-Target Pair
Affinity DataIC50: 220nMAssay Description:Inverse agonist activity at recombinant human GST-tagged ROR-gamma receptor ligand binding domain assessed as inhibition of receptor and co-activator...More data for this Ligand-Target Pair
Affinity DataIC50: 250nMAssay Description:In vivo inhibitory activity against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:In vitro inhibitory concentration against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:In vitro inhibitory concentration against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:In vitro inhibitory concentration against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:In vitro inhibitory concentration against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 350nMAssay Description:In vitro inhibitory concentration against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 350nMAssay Description:In vitro inhibitory concentration against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:In vitro inhibitory concentration against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:In vitro inhibitory concentration against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:In vitro inhibitory concentration against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:In vitro inhibitory concentration against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:In vitro inhibitory concentration against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:In vitro inhibitory concentration against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:In vivo inhibitory activity against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:In vivo inhibitory activity against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:In vivo inhibitory activity against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:In vivo inhibitory activity against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 450nMAssay Description:In vivo inhibitory activity against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 450nMAssay Description:In vitro inhibitory concentration against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 450nMAssay Description:In vitro inhibitory concentration against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:In vivo inhibitory activity against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:In vitro inhibitory concentration against human HeparanaseMore data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:Inhibition of Streptococcus pneumoniae acetyltransferase activity of GlmU using acetyl-CoA and glucosamine-1-phosphate after 30 mins by Ellman's meth...More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)