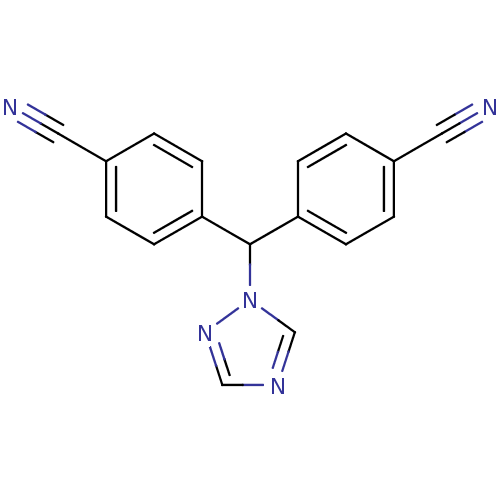
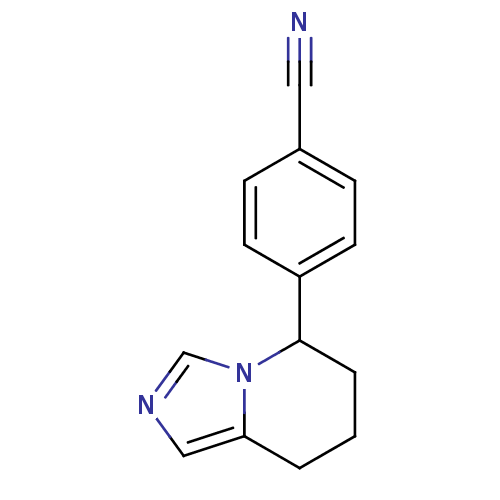
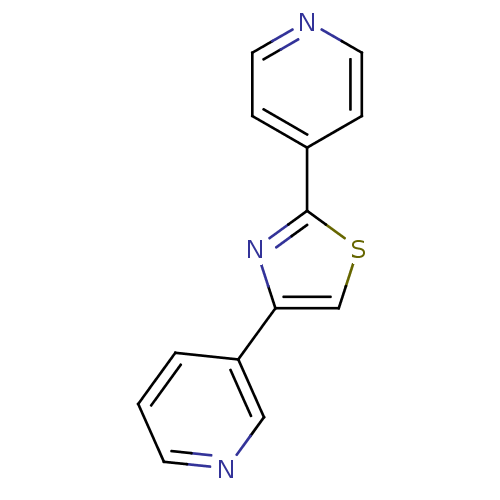
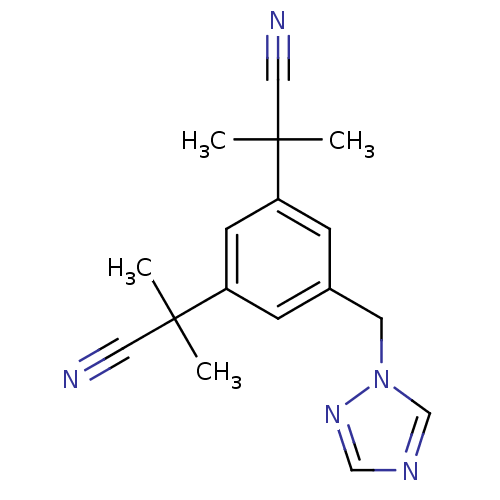
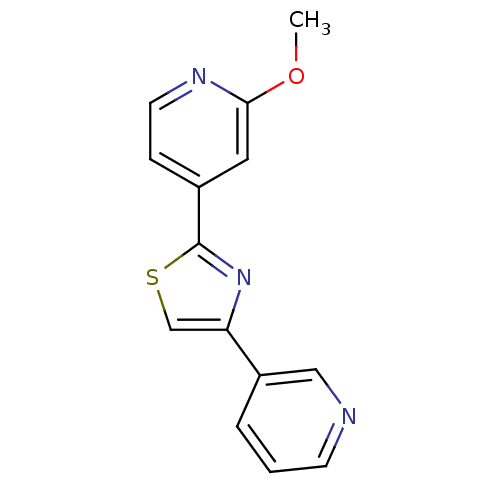
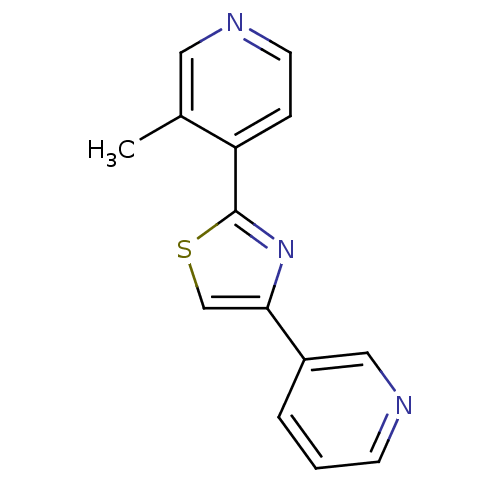
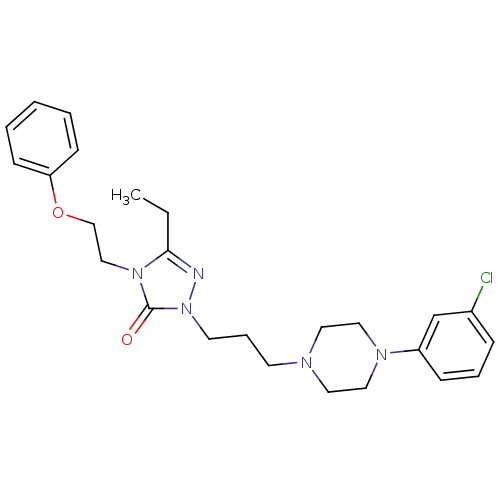
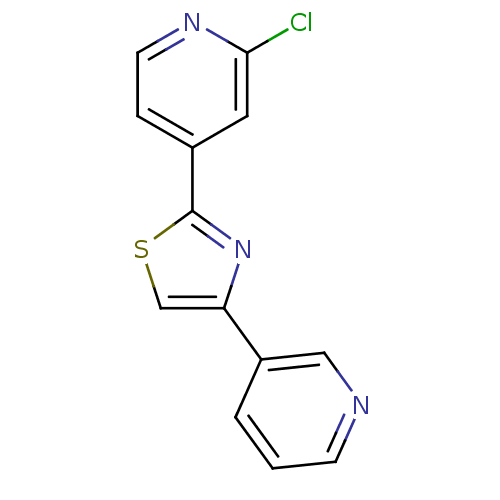
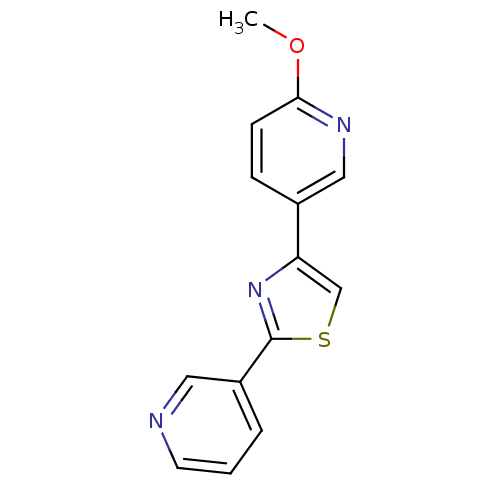
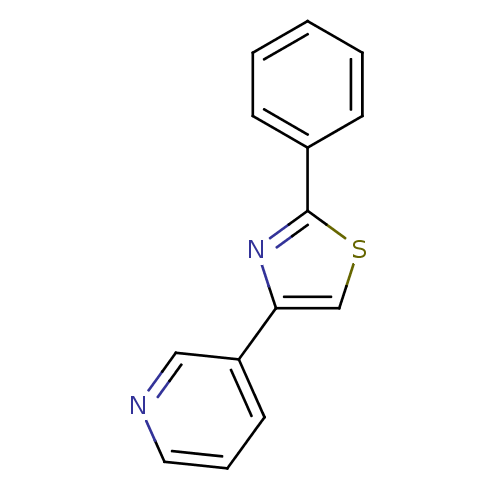
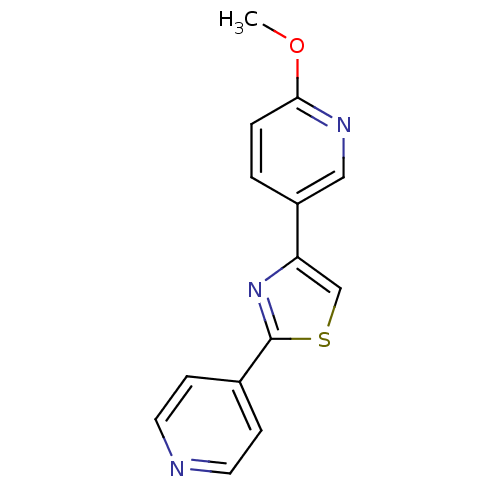
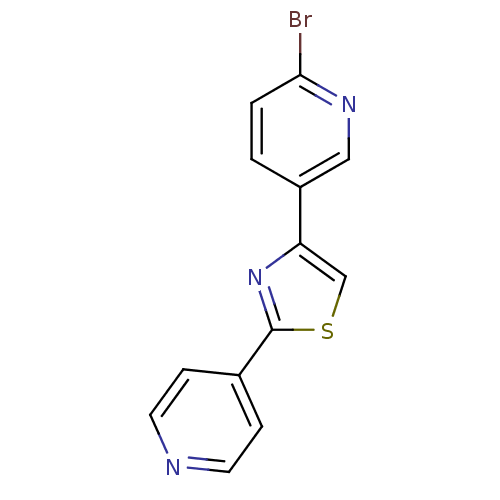
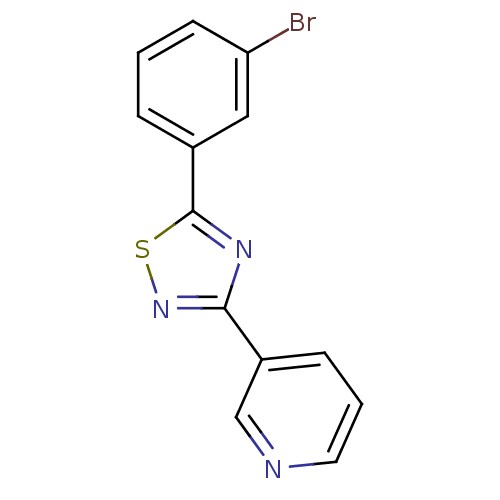
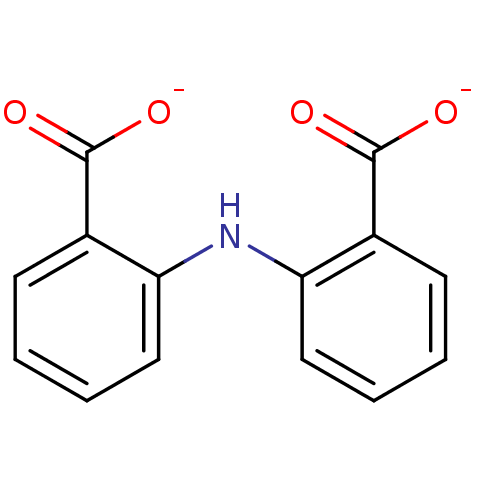
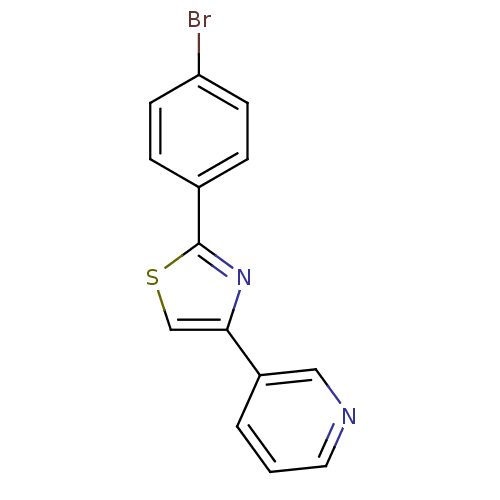
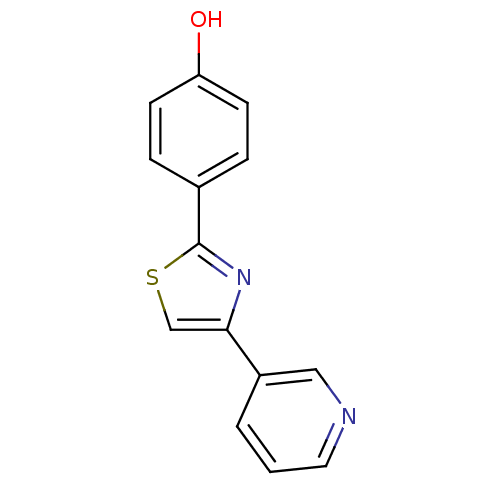
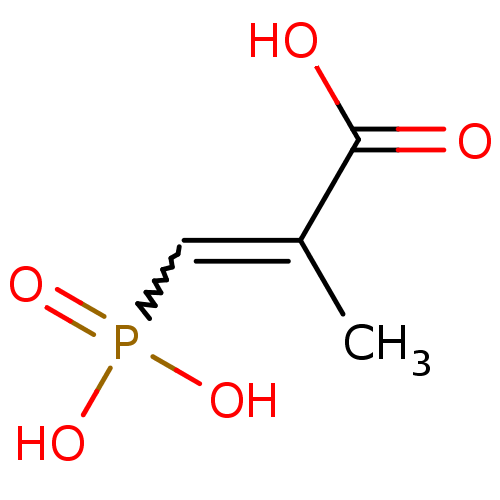
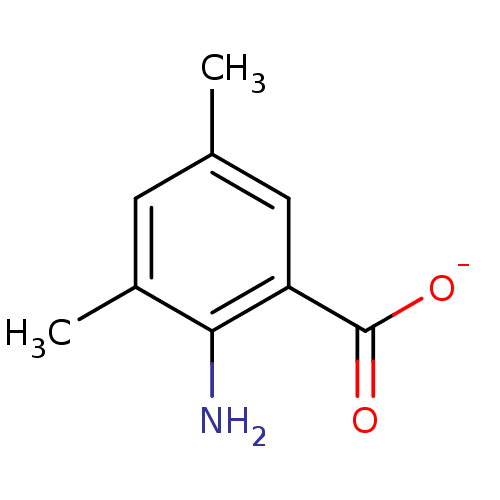
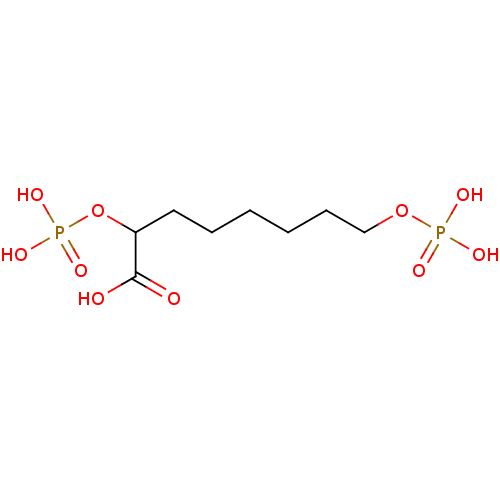
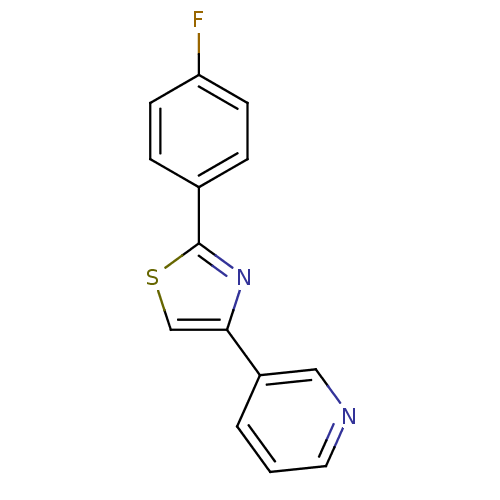
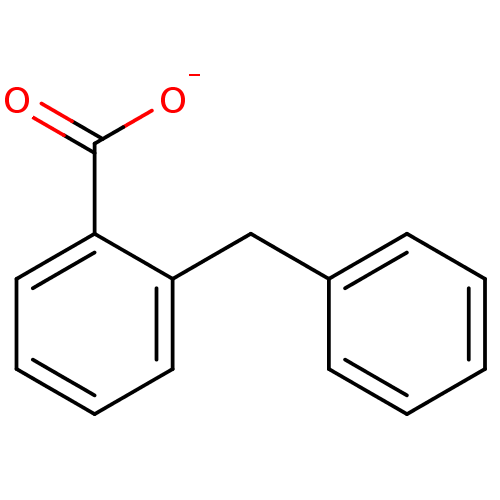
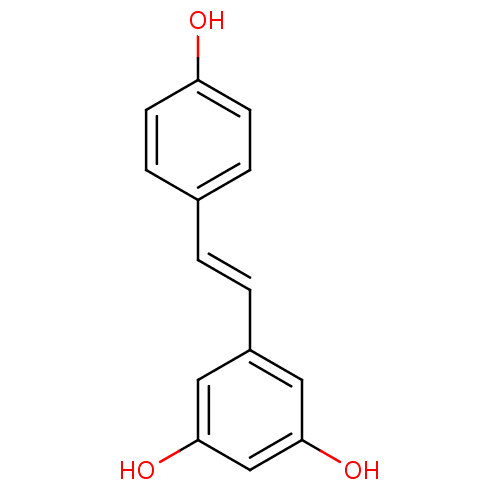
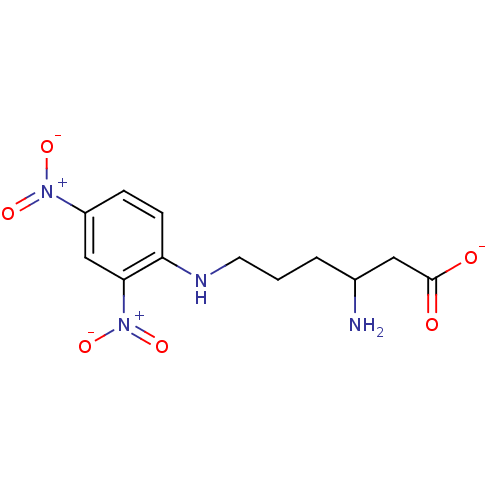
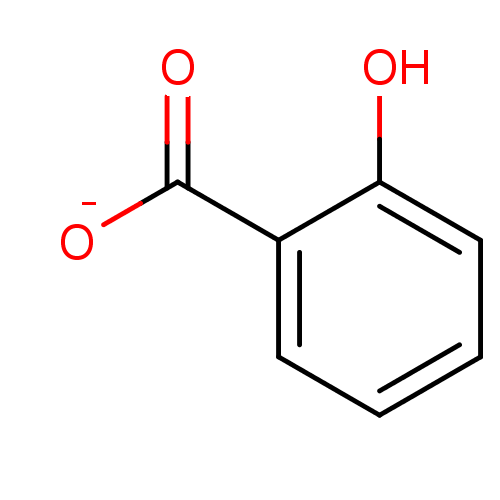
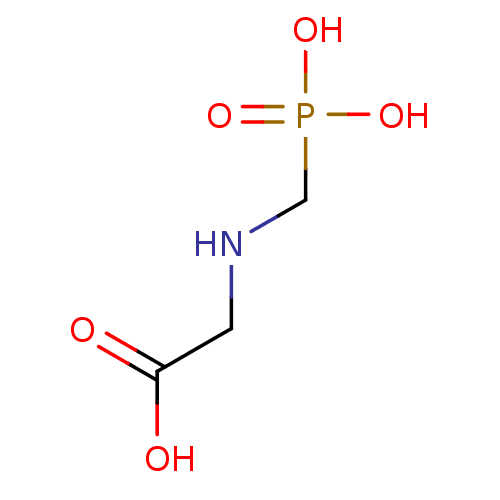
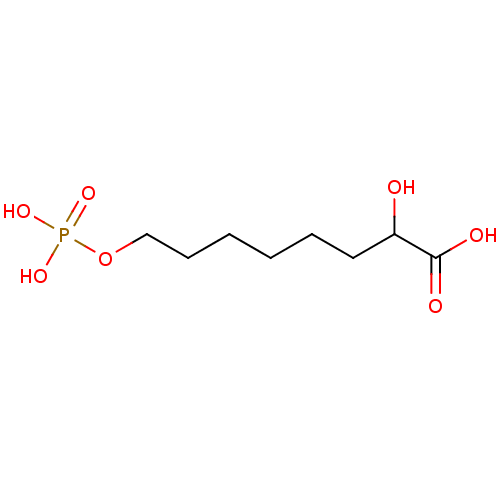
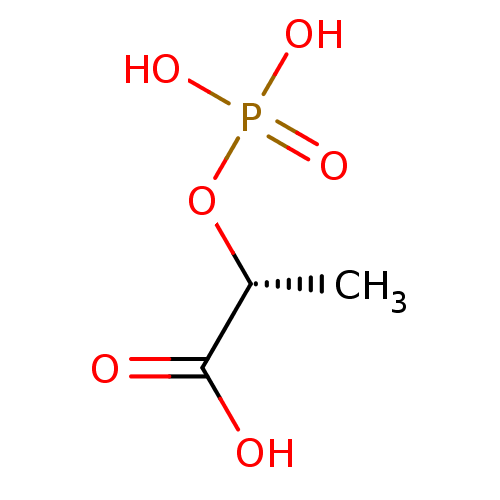
Affinity DataKi: 0.0200nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 0.0500nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 0.0600nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 0.130nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 0.650nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Binding affinity to 5-HT2AMore data for this Ligand-Target Pair
Affinity DataKi: 0.810nMAssay Description:Inhibition of 5-HT2A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.810nMAssay Description:Binding affinity to 5-HT2AMore data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:Binding affinity to SERTMore data for this Ligand-Target Pair
Affinity DataKi: 3.20nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 5.80nMAssay Description:Binding affinity to 5-HT2AMore data for this Ligand-Target Pair
Affinity DataKi: 8.20nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:Binding affinity to SERTMore data for this Ligand-Target Pair
Affinity DataKi: 35nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 63nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 83nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 86nMAssay Description:Inhibition of 5-HT2A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 86nMAssay Description:Binding affinity to 5-HT2AMore data for this Ligand-Target Pair
Affinity DataKi: 98nMAssay Description:Binding affinity to SERTMore data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 234nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 285nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 290nMAssay Description:Binding affinity to SERTMore data for this Ligand-Target Pair
Affinity DataKi: 1.16E+3nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 1.48E+3nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 1.50E+3nMAssay Description:The initial screen was performed in a 96-well plate using the fluoresence-based assay previously described. An enzyme-coupled absorbance-based assay...More data for this Ligand-Target Pair
Affinity DataKi: 1.72E+3nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 2.55E+3nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
TargetPhospho-2-dehydro-3-deoxyheptonate aldolase, Phe-sensitive(Escherichia coli (strain K12))
University Of Canterbury
Curated by ChEMBL
University Of Canterbury
Curated by ChEMBL
Affinity DataKi: 3.60E+3nMAssay Description:Competitive inhibition of recombinant Escherichia coli DAH7P synthase using PEP as substrate by Michaelis-Menten equation analysis using UV-visible s...More data for this Ligand-Target Pair
TargetPhospho-2-dehydro-3-deoxyheptonate aldolase, Phe-sensitive(Escherichia coli (strain K12))
University Of Canterbury
Curated by ChEMBL
University Of Canterbury
Curated by ChEMBL
Affinity DataKi: 4.70E+3nMAssay Description:Inhibition of recombinant Escherichia coli DAH7P synthaseMore data for this Ligand-Target Pair
TargetPhospho-2-dehydro-3-deoxyheptonate aldolase, Phe-sensitive(Escherichia coli (strain K12))
University Of Canterbury
Curated by ChEMBL
University Of Canterbury
Curated by ChEMBL
Affinity DataKi: 5.30E+3nMAssay Description:Competitive inhibition of recombinant Escherichia coli DAH7P synthase using PEP as substrate by Michaelis-Menten equation analysis using UV-visible s...More data for this Ligand-Target Pair
Affinity DataKi: 5.32E+3nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 6.30E+3nMAssay Description:The initial screen was performed in a 96-well plate using the fluoresence-based assay previously described. An enzyme-coupled absorbance-based assay...More data for this Ligand-Target Pair
Target2-dehydro-3-deoxyphosphooctonate aldolase(Neisseria meningitidis serogroup B (strain MC58))
University Of Canterbury
Curated by ChEMBL
University Of Canterbury
Curated by ChEMBL
Affinity DataKi: 7.90E+3nMAssay Description:Competitive inhibition of Neisseria meningitidis serotype B strain MC58 ATCC BAA355 KDO8P synthase expressed in Escherichia coli BL21 (DE3) cells usi...More data for this Ligand-Target Pair
Affinity DataKi: 1.26E+4nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 1.50E+4nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 1.50E+4nMAssay Description:The initial screen was performed in a 96-well plate using the fluoresence-based assay previously described. An enzyme-coupled absorbance-based assay...More data for this Ligand-Target Pair
Affinity DataKi: 1.80E+4nMAssay Description:The initial screen was performed in a 96-well plate using the fluoresence-based assay previously described. An enzyme-coupled absorbance-based assay...More data for this Ligand-Target Pair
Affinity DataKi: 1.90E+4nMAssay Description:The initial screen was performed in a 96-well plate using the fluoresence-based assay previously described. An enzyme-coupled absorbance-based assay...More data for this Ligand-Target Pair
Affinity DataKi: 4.17E+4nMAssay Description:Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael...More data for this Ligand-Target Pair
Affinity DataKi: 6.00E+4nMAssay Description:The initial screen was performed in a 96-well plate using the fluoresence-based assay previously described. An enzyme-coupled absorbance-based assay...More data for this Ligand-Target Pair
Affinity DataKi: 7.90E+4nMAssay Description:The initial screen was performed in a 96-well plate using the fluoresence-based assay previously described. An enzyme-coupled absorbance-based assay...More data for this Ligand-Target Pair
Affinity DataKi: 1.19E+5nMAssay Description:The initial screen was performed in a 96-well plate using the fluoresence-based assay previously described. An enzyme-coupled absorbance-based assay...More data for this Ligand-Target Pair
TargetPhospho-2-dehydro-3-deoxyheptonate aldolase, Phe-sensitive(Escherichia coli (strain K12))
University Of Canterbury
Curated by ChEMBL
University Of Canterbury
Curated by ChEMBL
Affinity DataKi: 1.54E+5nMAssay Description:Competitive inhibition of recombinant Escherichia coli DAH7P synthase using PEP as substrate by Michaelis-Menten equation analysis using UV-visible s...More data for this Ligand-Target Pair
Target2-dehydro-3-deoxyphosphooctonate aldolase(Neisseria meningitidis serogroup B (strain MC58))
University Of Canterbury
Curated by ChEMBL
University Of Canterbury
Curated by ChEMBL
Affinity DataKi: 2.60E+5nMAssay Description:Competitive inhibition of Neisseria meningitidis serotype B strain MC58 ATCC BAA355 KDO8P synthase expressed in Escherichia coli BL21 (DE3) cells usi...More data for this Ligand-Target Pair
TargetPhospho-2-dehydro-3-deoxyheptonate aldolase, Phe-sensitive(Escherichia coli (strain K12))
University Of Canterbury
Curated by ChEMBL
University Of Canterbury
Curated by ChEMBL
Affinity DataKi: 2.70E+5nMAssay Description:Inhibition of recombinant Escherichia coli DAH7P synthaseMore data for this Ligand-Target Pair
Target2-dehydro-3-deoxyphosphooctonate aldolase(Neisseria meningitidis serogroup B (strain MC58))
University Of Canterbury
Curated by ChEMBL
University Of Canterbury
Curated by ChEMBL
Affinity DataKi: 2.70E+5nMAssay Description:Competitive inhibition of Neisseria meningitidis serotype B strain MC58 ATCC BAA355 KDO8P synthase expressed in Escherichia coli BL21 (DE3) cells usi...More data for this Ligand-Target Pair
Target2-dehydro-3-deoxyphosphooctonate aldolase(Neisseria meningitidis serogroup B (strain MC58))
University Of Canterbury
Curated by ChEMBL
University Of Canterbury
Curated by ChEMBL
Affinity DataKi: 8.70E+5nMAssay Description:Competitive inhibition of Neisseria meningitidis serotype B strain MC58 ATCC BAA355 KDO8P synthase expressed in Escherichia coli BL21 (DE3) cells usi...More data for this Ligand-Target Pair