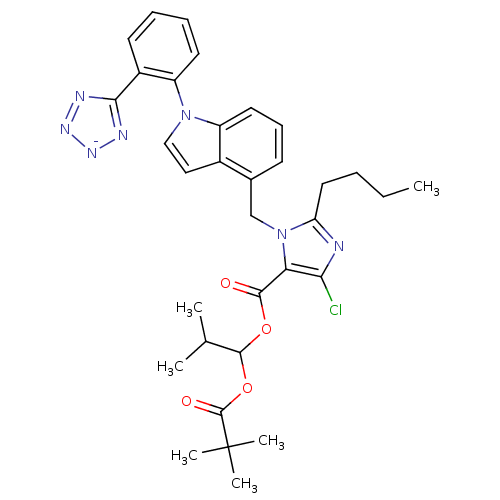
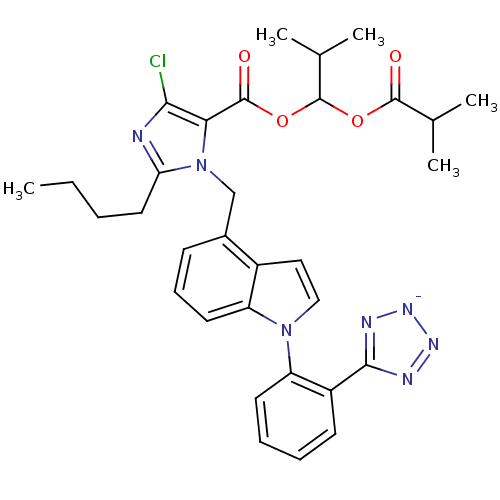
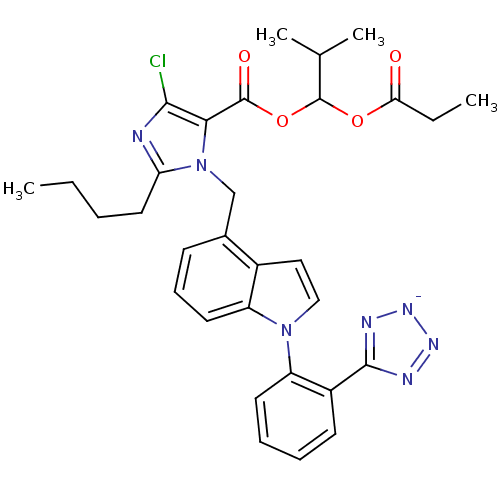
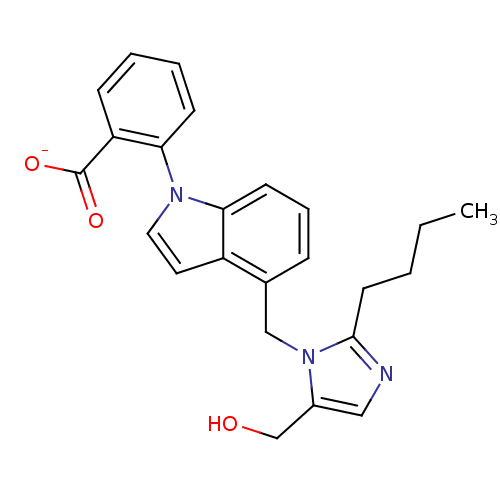
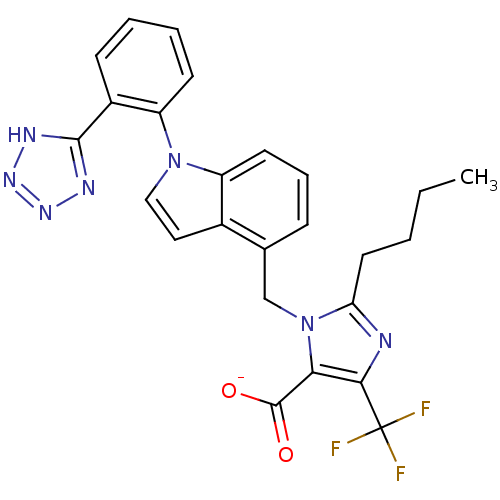
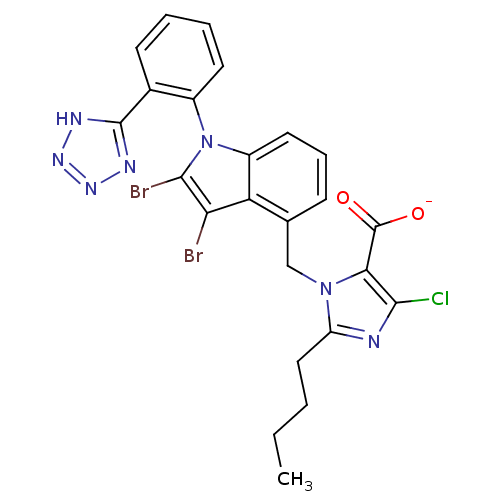
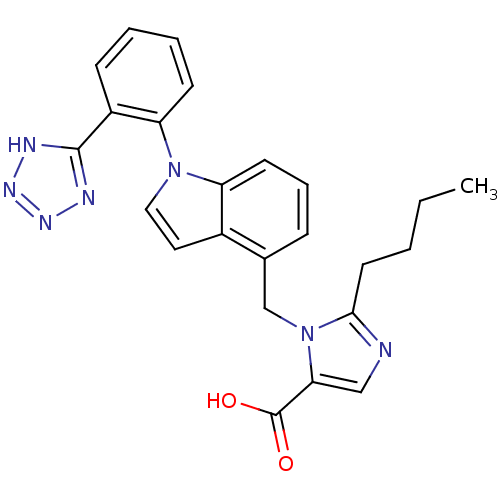
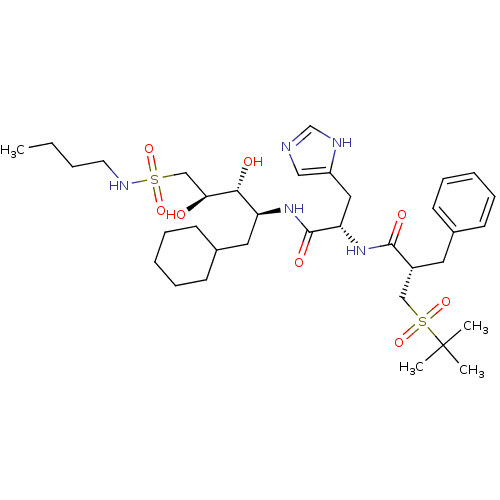
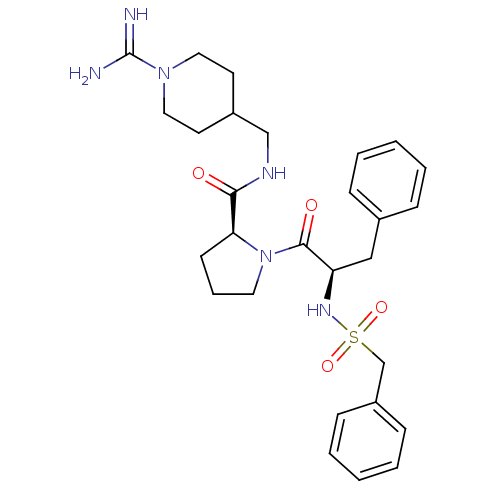
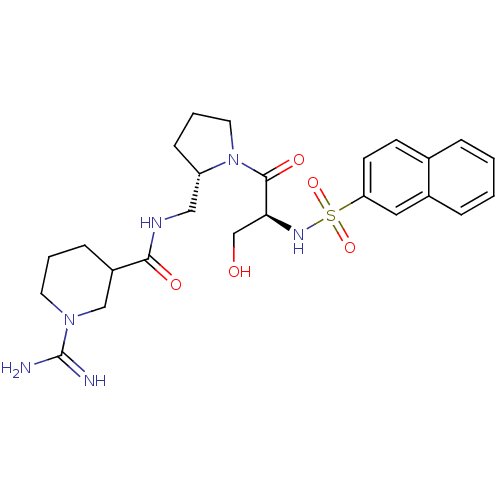
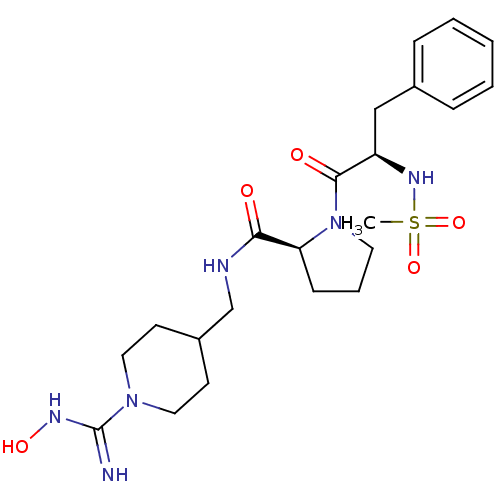
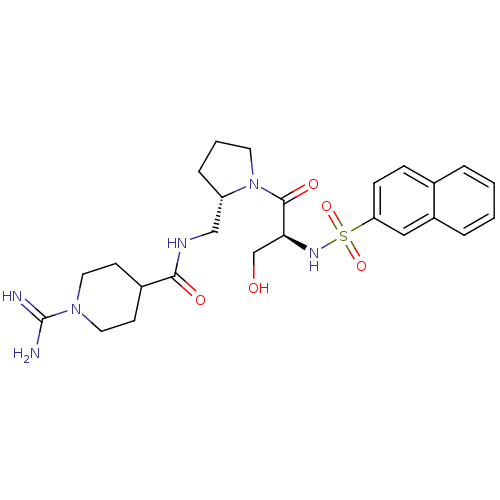
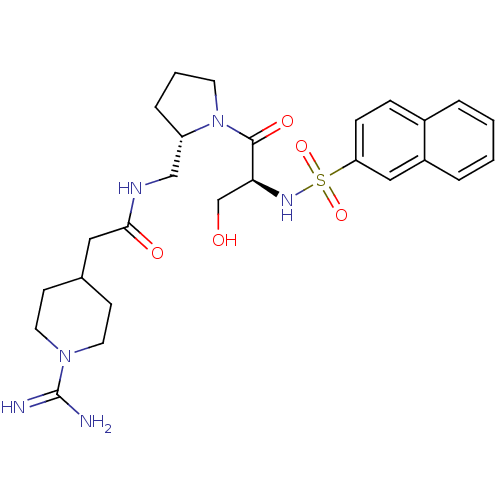
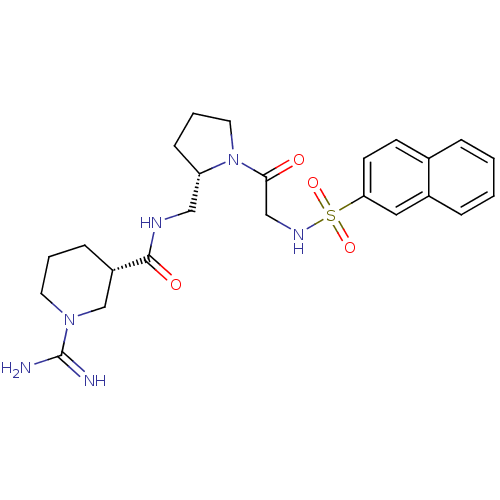
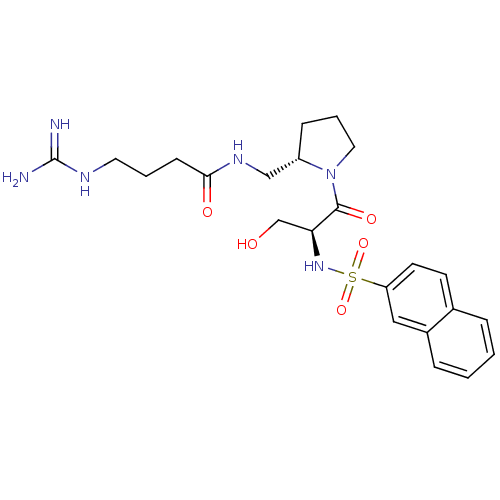
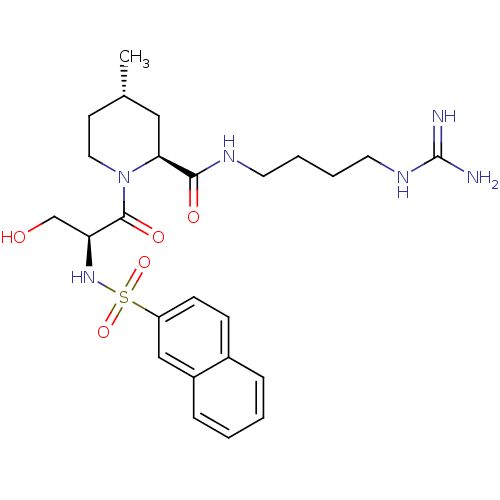
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.460nMAssay Description:Competitive kinetic for thrombin inhibition Ki was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranesMore data for this Ligand-Target Pair
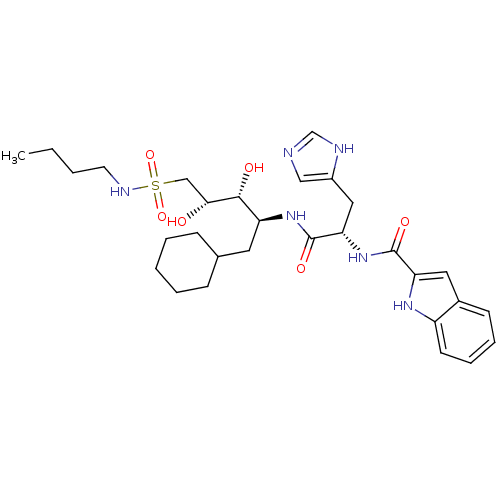
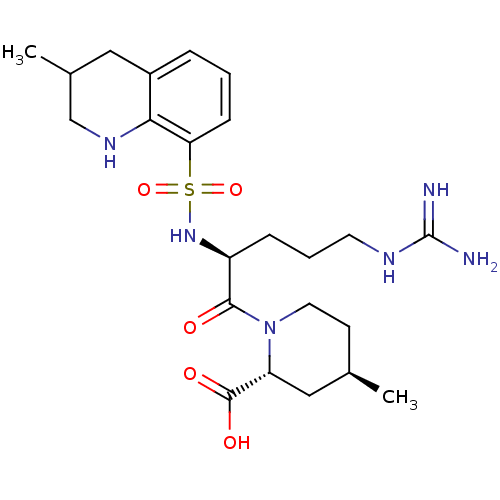
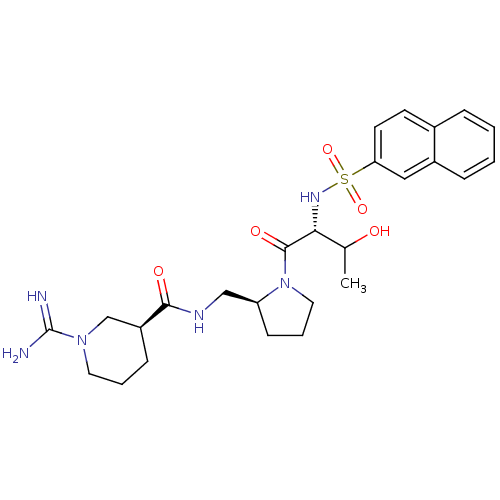
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 3.40nMAssay Description:In vitro reversible inhibition of thrombin catalytic activityMore data for this Ligand-Target Pair
Affinity DataKi: 4.5nMAssay Description:Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 6.30nMAssay Description:Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 6.30nMAssay Description:Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 7.30nMAssay Description:Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranesMore data for this Ligand-Target Pair
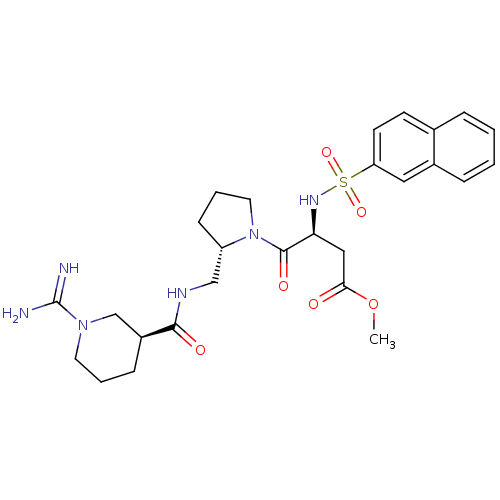
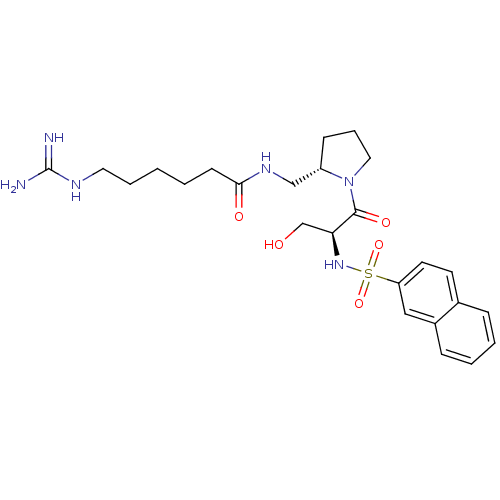
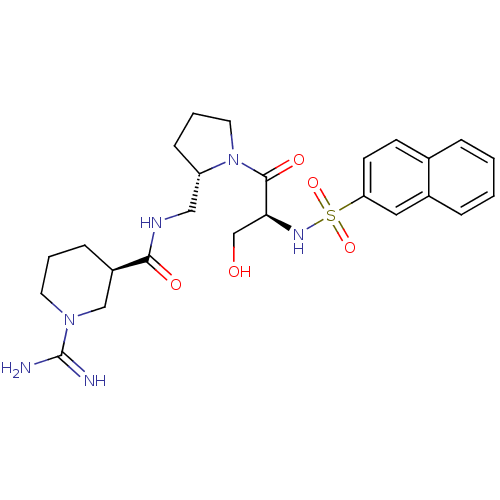
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 8.20nMAssay Description:Competitive kinetic for human alpha thrombin inhibition Ki was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 8.90nMAssay Description:Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 39nMAssay Description:Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 79nMAssay Description:Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 79nMAssay Description:Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 760nMAssay Description:Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 840nMAssay Description:Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 3.60E+3nMAssay Description:Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 6.10E+3nMAssay Description:Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 6.50E+3nMAssay Description:Receptor binding affinity for Angiotensin II receptor, type 2 determinedMore data for this Ligand-Target Pair
Affinity DataIC50: 0.350nMpH: 7.0Assay Description:The compound was tested for its inhibitory activity against human renal renin at pH 7More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMpH: 7.0Assay Description:The compound was tested for its inhibitory activity against human renal renin at pH 7More data for this Ligand-Target Pair
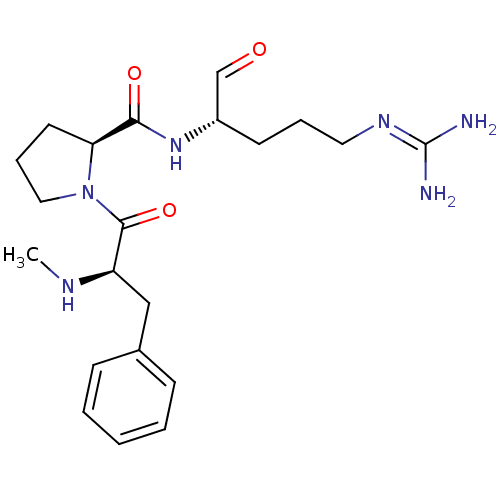
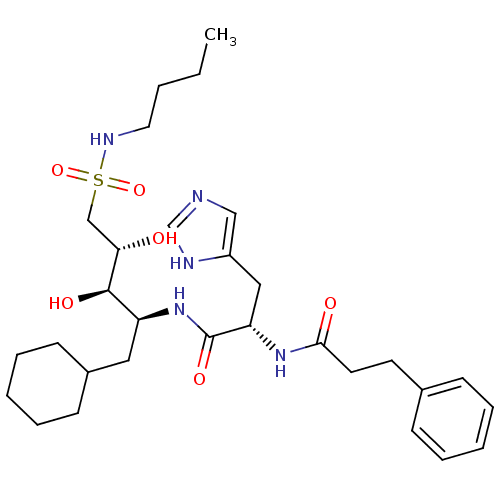
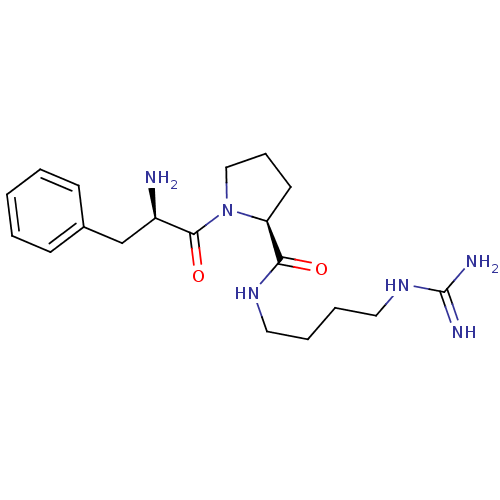
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:In vitro inhibitory activity against hydrolysis of thrombin was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMpH: 7.0Assay Description:The compound was tested for its inhibitory activity against human renal renin at pH 7More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation.More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation.More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation.More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation.More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rt after 3 min incubation with compoundMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 32nMAssay Description:In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation.More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 38nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 38nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rat after 3 min incubation with compoundMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 46nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation.More data for this Ligand-Target Pair
Affinity DataIC50: 64nMpH: 7.0Assay Description:The compound was tested for its inhibitory activity against human renal renin at pH 7More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 75nMAssay Description:In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation.More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 80nMAssay Description:In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation.More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 110nMAssay Description:In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation.More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation.More data for this Ligand-Target Pair
Affinity DataIC50: 240nMpH: 7.0Assay Description:The compound was tested for its inhibitory activity against human renal renin at pH 7More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 310nMAssay Description:In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation.More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 390nMAssay Description:In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation.More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 500nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rat after 3 min incubation with compoundMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 500nMAssay Description:In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation.More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 540nMAssay Description:In vitro inhibitory activity against hydrolysis of human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 620nMAssay Description:In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation.More data for this Ligand-Target Pair

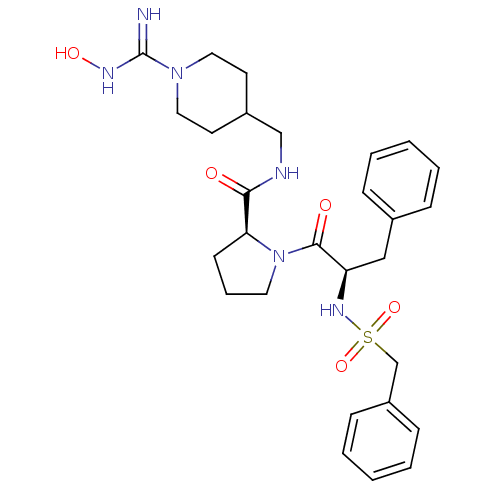

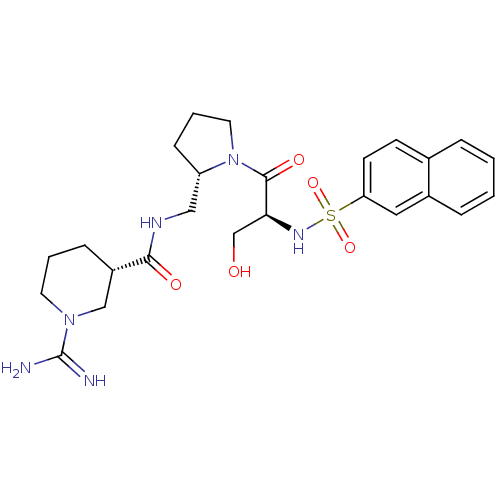
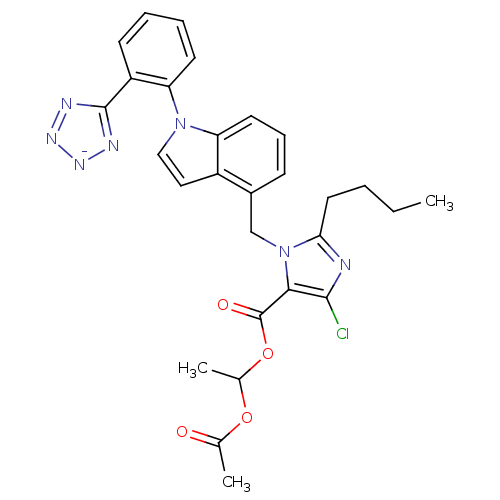
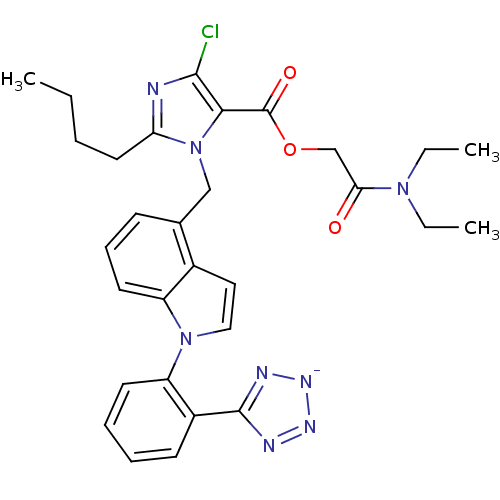
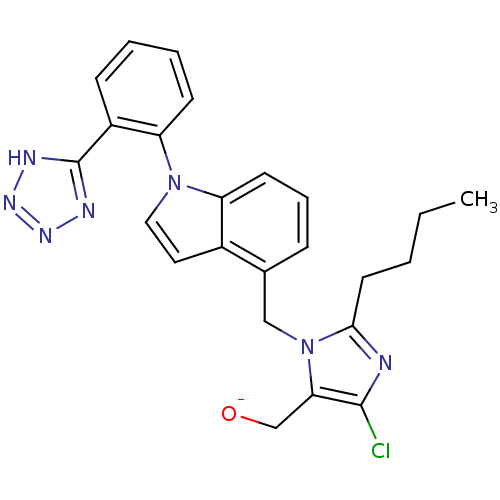

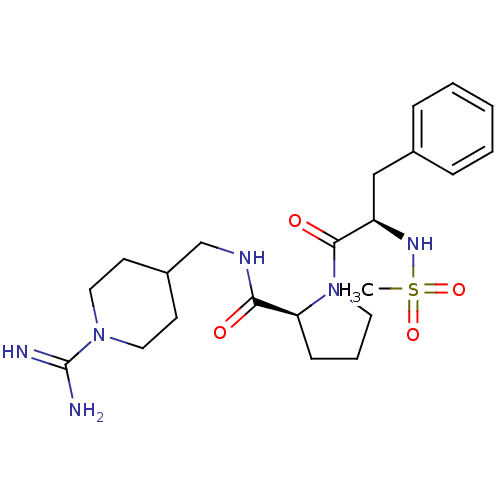
 3D Structure (crystal)
3D Structure (crystal)