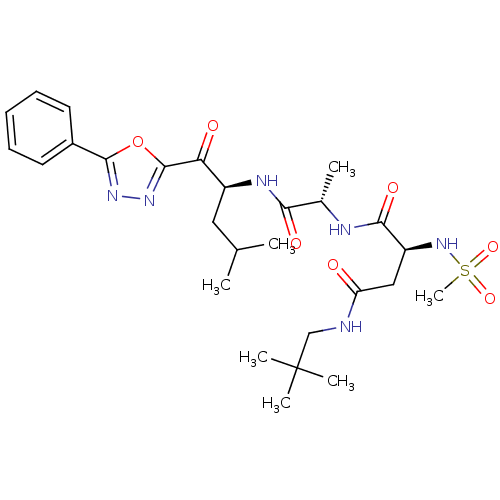
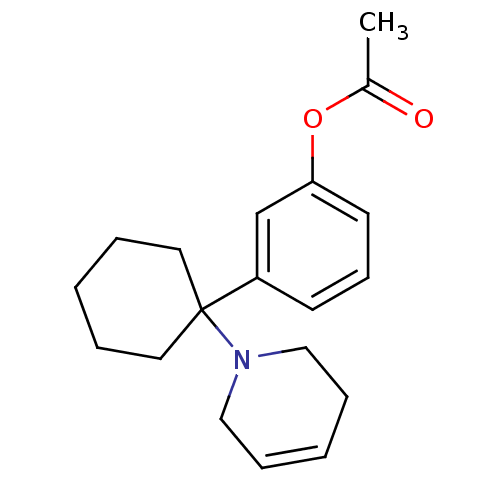
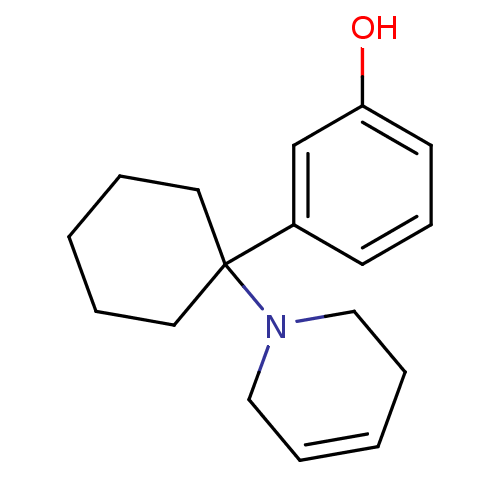
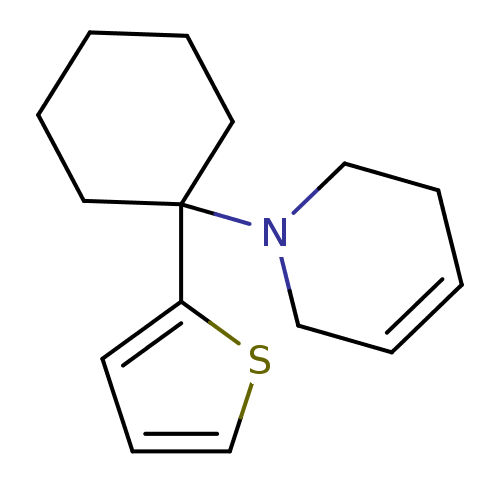
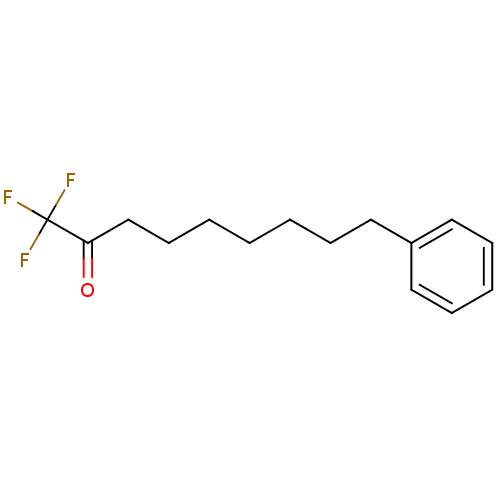
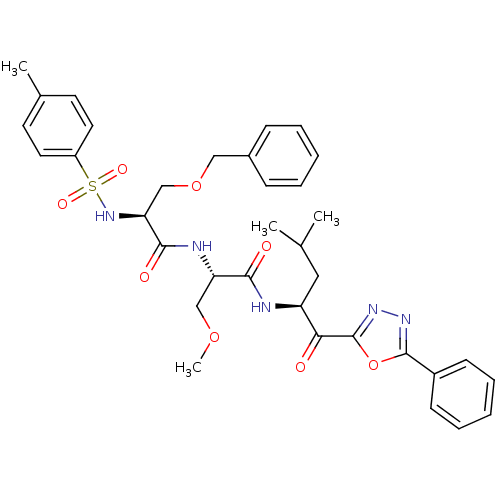
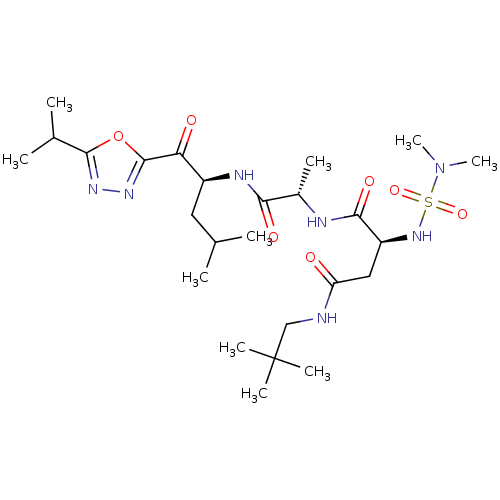
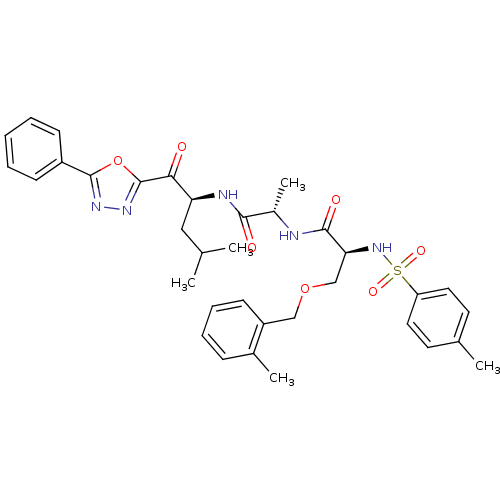
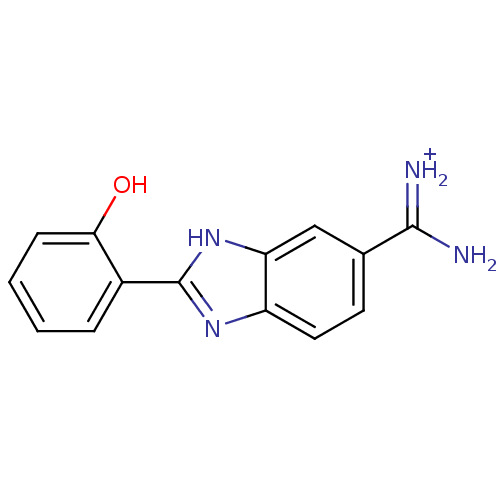
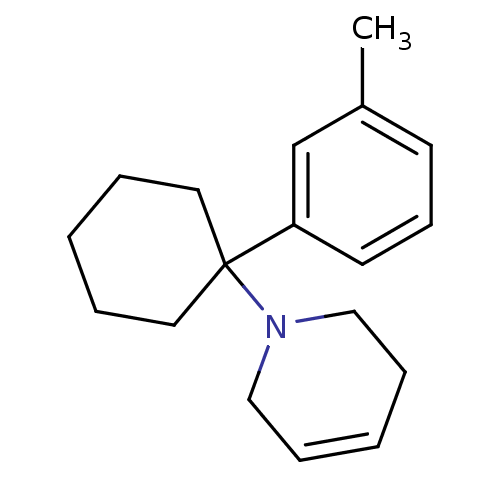
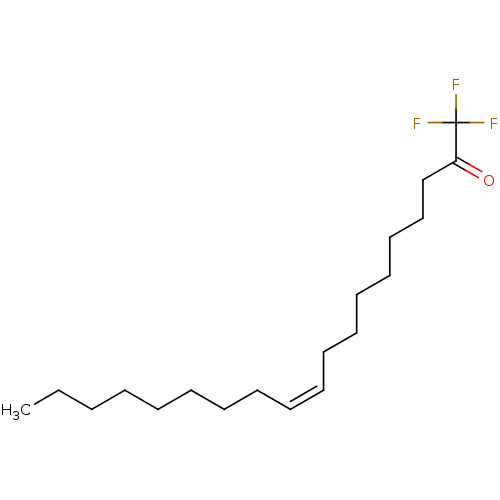
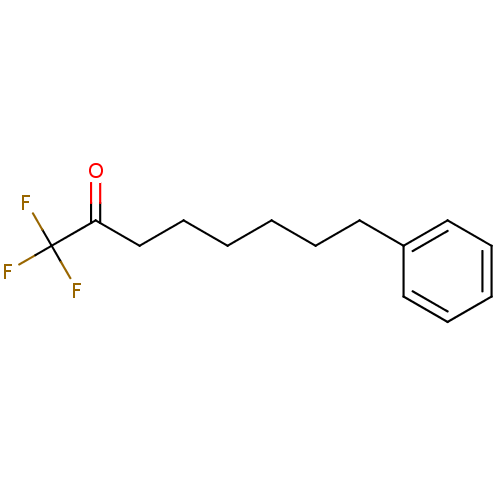
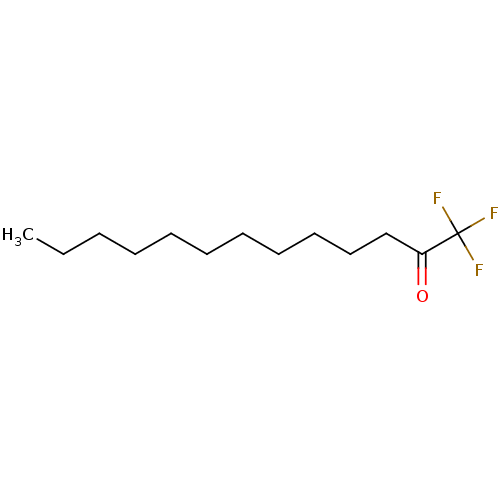
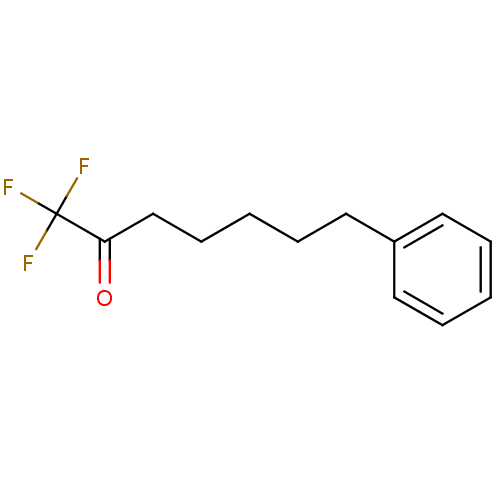
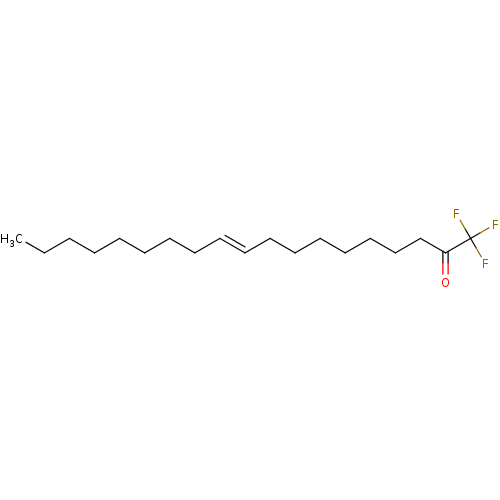
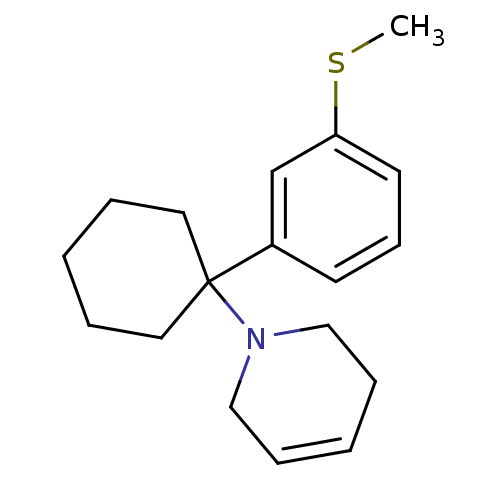
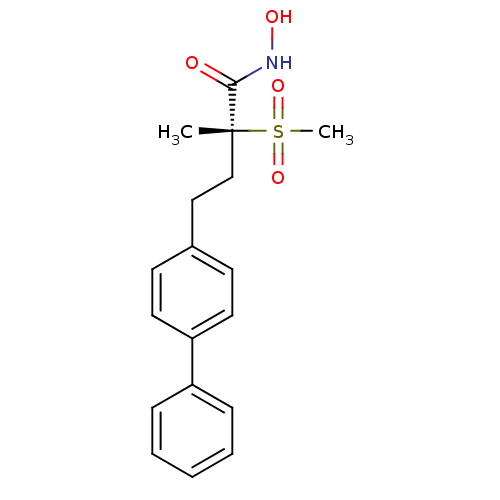
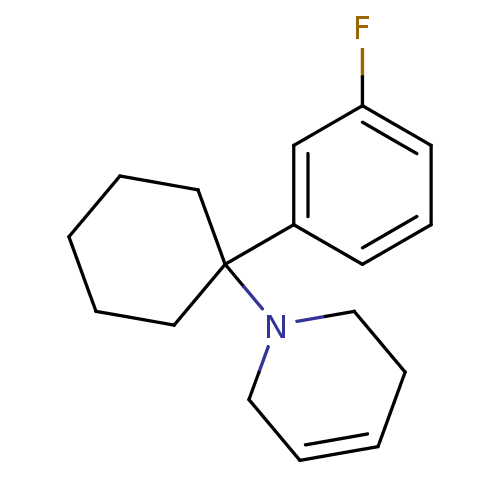
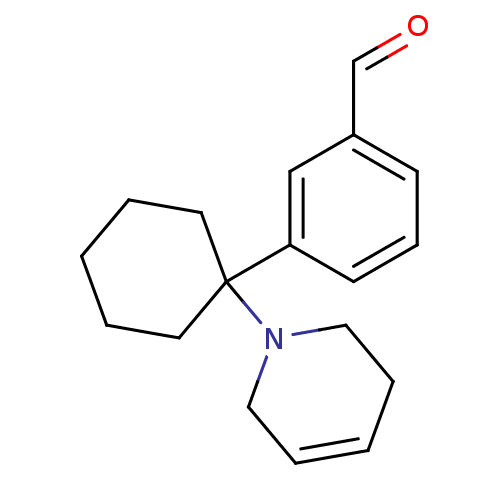
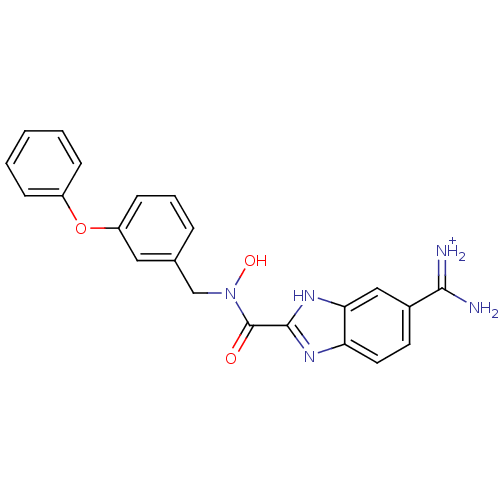
Affinity DataKi: 0.5nMAssay Description:Inhibition of chymotrypsin-like proteasome activity of human 20S proteasomeMore data for this Ligand-Target Pair
Affinity DataKi: 0.510nMAssay Description:Inhibition of chymotrypsin-like proteasome activity of human 20S proteasomeMore data for this Ligand-Target Pair
Affinity DataKi: 0.650nMAssay Description:Inhibition of chymotrypsin-like proteasome activity of human 20S proteasomeMore data for this Ligand-Target Pair
Affinity DataKi: 0.720nMAssay Description:Inhibition of chymotrypsin-like proteasome activity of human 20S proteasomeMore data for this Ligand-Target Pair
Affinity DataKi: 0.720nMAssay Description:Inhibition of chymotrypsin-like proteasome activity of human 20S proteasomeMore data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:Inhibition of chymotrypsin-like proteasome activity of human 20S proteasomeMore data for this Ligand-Target Pair
Affinity DataKi: 3.70nMAssay Description:Inhibition of chymotrypsin-like proteasome activity of human 20S proteasomeMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Binding affinity towards recombinant thymidine phosphorylase TPMore data for this Ligand-Target Pair
Affinity DataKi: 5.70nMAssay Description:Inhibition of chymotrypsin-like proteasome activity of human 20S proteasomeMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2C(Rattus norvegicus (Rat))
National Institute Of Diabetes
Curated by ChEMBL
National Institute Of Diabetes
Curated by ChEMBL
Affinity DataKi: 6nMAssay Description:Ability to displace [3H]- TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum.More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2C(Rattus norvegicus (Rat))
National Institute Of Diabetes
Curated by ChEMBL
National Institute Of Diabetes
Curated by ChEMBL
Affinity DataKi: 7nMAssay Description:Ability to displace [3H]-TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum.More data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2C(Rattus norvegicus (Rat))
National Institute Of Diabetes
Curated by ChEMBL
National Institute Of Diabetes
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:Ability to displace [3H]-TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum.More data for this Ligand-Target Pair
Affinity DataKi: 17nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
TargetFatty-acid amide hydrolase 1 [30-579](Rattus norvegicus (rat))
Scripps Research Institute
Curated by ChEMBL
Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 25nMAssay Description:Inhibitory concentration of the compound against Fatty-acid amide hydrolase (FAAH) in rat liverMore data for this Ligand-Target Pair
Affinity DataKi: 32nMAssay Description:Inhibition of chymotrypsin-like proteasome activity of human 20S proteasomeMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2C(Rattus norvegicus (Rat))
National Institute Of Diabetes
Curated by ChEMBL
National Institute Of Diabetes
Curated by ChEMBL
Affinity DataKi: 33nMAssay Description:Ability to displace [3H]-TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum.More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2C(Rattus norvegicus (Rat))
National Institute Of Diabetes
Curated by ChEMBL
National Institute Of Diabetes
Curated by ChEMBL
Affinity DataKi: 37nMAssay Description:Ability to displace [3H]- TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum.More data for this Ligand-Target Pair
Affinity DataKi: 38nMAssay Description:Inhibition of chymotrypsin-like proteasome activity of human 20S proteasomeMore data for this Ligand-Target Pair
Affinity DataKi: 43nM ΔG°: -41.6kJ/molepH: 8.0 T: 2°CAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 45nMAssay Description:Inhibition of chymotrypsin-like proteasome activity of human 20S proteasomeMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2C(Rattus norvegicus (Rat))
National Institute Of Diabetes
Curated by ChEMBL
National Institute Of Diabetes
Curated by ChEMBL
Affinity DataKi: 56nMAssay Description:Ability to displace [3H]-TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum.More data for this Ligand-Target Pair
Affinity DataKi: 65nM ΔG°: -40.6kJ/molepH: 8.0 T: 2°CAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2C(Rattus norvegicus (Rat))
National Institute Of Diabetes
Curated by ChEMBL
National Institute Of Diabetes
Curated by ChEMBL
Affinity DataKi: 81nMAssay Description:Ability to displace [3H]- TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum.More data for this Ligand-Target Pair
TargetFatty-acid amide hydrolase 1 [30-579](Rattus norvegicus (rat))
Scripps Research Institute
Curated by ChEMBL
Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 82nMAssay Description:Inhibitory concentration of the compound against Fatty-acid amide hydrolase (FAAH) in rat liverMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2C(Rattus norvegicus (Rat))
National Institute Of Diabetes
Curated by ChEMBL
National Institute Of Diabetes
Curated by ChEMBL
Affinity DataKi: 92nMAssay Description:Ability to displace [3H]- TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum.More data for this Ligand-Target Pair
TargetFatty-acid amide hydrolase 1 [30-579](Rattus norvegicus (rat))
Scripps Research Institute
Curated by ChEMBL
Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 96nMAssay Description:Inhibitory constant of the compound against Fatty-acid amide hydrolase (FAAH) in rat liverMore data for this Ligand-Target Pair
TargetFatty-acid amide hydrolase 1 [30-579](Rattus norvegicus (rat))
Scripps Research Institute
Curated by ChEMBL
Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 100nMAssay Description:Inhibitory constant of the compound against Fatty-acid amide hydrolase (FAAH) in rat liverMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2C(Rattus norvegicus (Rat))
National Institute Of Diabetes
Curated by ChEMBL
National Institute Of Diabetes
Curated by ChEMBL
Affinity DataKi: 114nMAssay Description:Ability to displace [3H]- TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum.More data for this Ligand-Target Pair
Affinity DataKi: 130nMAssay Description:Inhibition of chymotrypsin-like proteasome activity of human 20S proteasomeMore data for this Ligand-Target Pair
TargetFatty-acid amide hydrolase 1 [30-579](Rattus norvegicus (rat))
Scripps Research Institute
Curated by ChEMBL
Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 130nMAssay Description:Inhibitory constant of the compound against Fatty-acid amide hydrolase (FAAH) in rat liverMore data for this Ligand-Target Pair
TargetFatty-acid amide hydrolase 1 [30-579](Rattus norvegicus (rat))
Scripps Research Institute
Curated by ChEMBL
Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 140nMAssay Description:Inhibitory constant of the compound against Fatty-acid amide hydrolase (FAAH) in rat liverMore data for this Ligand-Target Pair
Affinity DataKi: 140nMAssay Description:Inhibition of chymotrypsin-like proteasome activity of human 20S proteasomeMore data for this Ligand-Target Pair
TargetFatty-acid amide hydrolase 1 [30-579](Rattus norvegicus (rat))
Scripps Research Institute
Curated by ChEMBL
Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 140nMAssay Description:Inhibitory constant of the compound against Fatty-acid amide hydrolase (FAAH) in rat liverMore data for this Ligand-Target Pair
TargetFatty-acid amide hydrolase 1 [30-579](Rattus norvegicus (rat))
Scripps Research Institute
Curated by ChEMBL
Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 170nMAssay Description:Inhibitory concentration of the compound against Fatty-acid amide hydrolase (FAAH) in rat liverMore data for this Ligand-Target Pair
Affinity DataKi: 170nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
TargetFatty-acid amide hydrolase 1 [30-579](Rattus norvegicus (rat))
Scripps Research Institute
Curated by ChEMBL
Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 170nMAssay Description:Inhibitory constant of the compound against Fatty-acid amide hydrolase (FAAH) in rat liverMore data for this Ligand-Target Pair
TargetFatty-acid amide hydrolase 1(Homo sapiens (Human))
Institute For Chemical Biology
Curated by ChEMBL
Institute For Chemical Biology
Curated by ChEMBL
Affinity DataKi: 190nMpH: 9.0Assay Description:Compound was tested for inhibitory potency against Fatty-acid amide hydrolase (FAAH) at pH 9.0More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2C(Rattus norvegicus (Rat))
National Institute Of Diabetes
Curated by ChEMBL
National Institute Of Diabetes
Curated by ChEMBL
Affinity DataKi: 231nMAssay Description:Ability to displace [3H]- TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum.More data for this Ligand-Target Pair
Affinity DataKi: 236nMAssay Description:Inhibition of human thymidine phosphorylase by continuous spectrophotometric assayMore data for this Ligand-Target Pair
TargetFatty-acid amide hydrolase 1 [30-579](Rattus norvegicus (rat))
Scripps Research Institute
Curated by ChEMBL
Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 240nMAssay Description:Inhibitory constant of the compound against Fatty-acid amide hydrolase (FAAH) in rat liverMore data for this Ligand-Target Pair
Affinity DataKi: 250nMAssay Description:Inhibition of human norepinephrine transporterMore data for this Ligand-Target Pair
TargetFatty-acid amide hydrolase 1(Homo sapiens (Human))
Institute For Chemical Biology
Curated by ChEMBL
Institute For Chemical Biology
Curated by ChEMBL
Affinity DataKi: 250nMpH: 9.0Assay Description:Compound was tested for inhibitory potency against Fatty-acid amide hydrolase (FAAH) at pH 9.0More data for this Ligand-Target Pair
Affinity DataKi: 280nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2C(Rattus norvegicus (Rat))
National Institute Of Diabetes
Curated by ChEMBL
National Institute Of Diabetes
Curated by ChEMBL
Affinity DataKi: 281nMAssay Description:Ability to displace [3H]- TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum.More data for this Ligand-Target Pair
TargetFatty-acid amide hydrolase 1 [30-579](Rattus norvegicus (rat))
Scripps Research Institute
Curated by ChEMBL
Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 320nMAssay Description:Inhibitory constant of the compound against Fatty-acid amide hydrolase (FAAH) in rat liverMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2C(Rattus norvegicus (Rat))
National Institute Of Diabetes
Curated by ChEMBL
National Institute Of Diabetes
Curated by ChEMBL
Affinity DataKi: 324nMAssay Description:Ability to displace [3H]- TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum.More data for this Ligand-Target Pair
Affinity DataKi: 330nMAssay Description:Inhibition of trypsin-like proteasome activity of human 20S proteasomeMore data for this Ligand-Target Pair
Affinity DataKi: 340nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 380nM ΔG°: -36.3kJ/molepH: 7.7 T: 2°CAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair


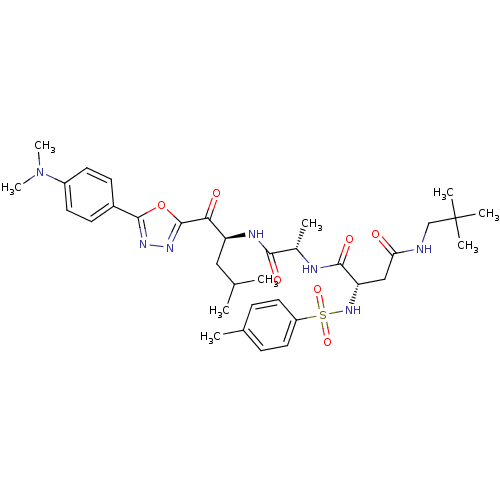
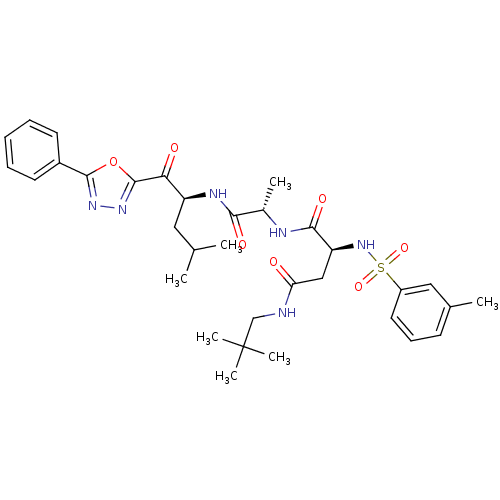
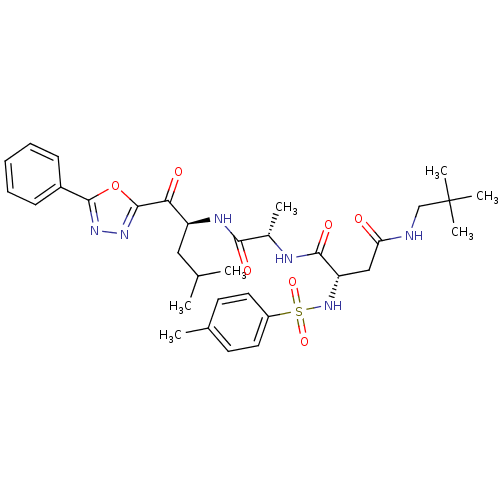
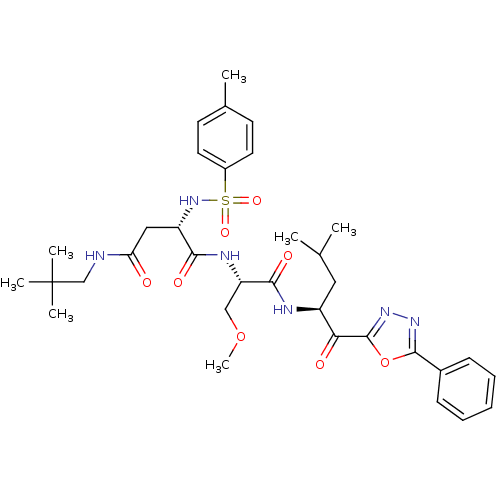

 3D Structure (crystal)
3D Structure (crystal)