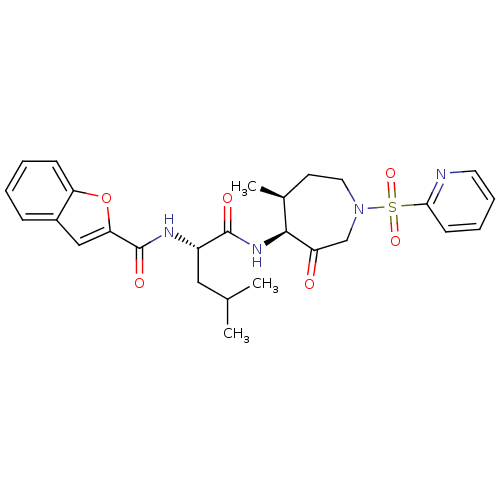
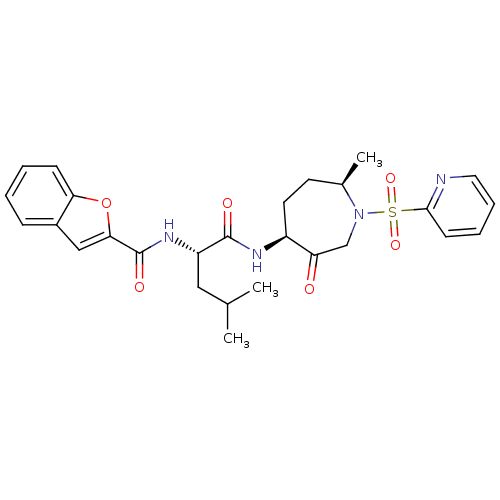
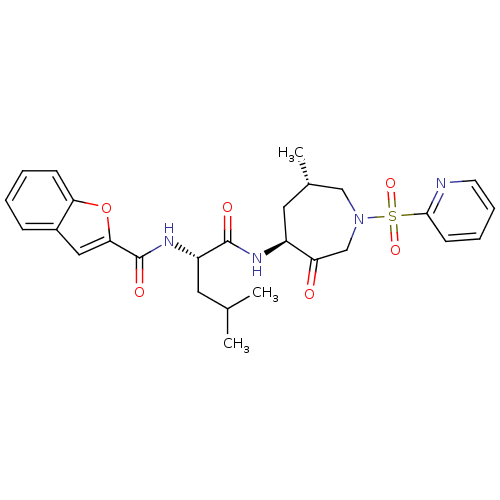
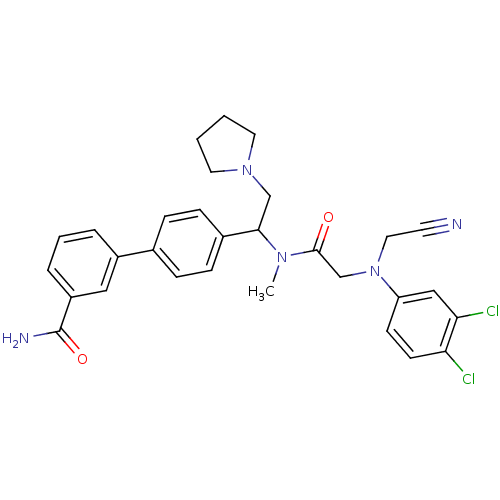
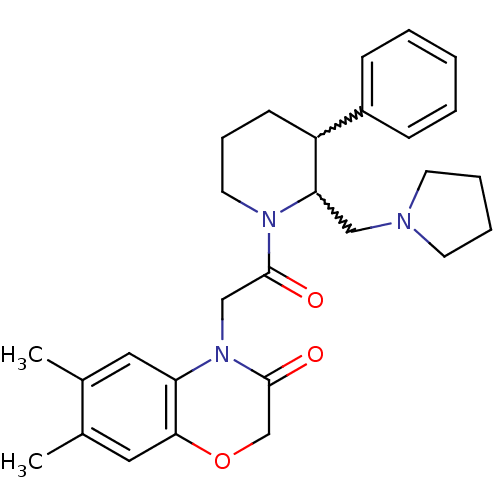
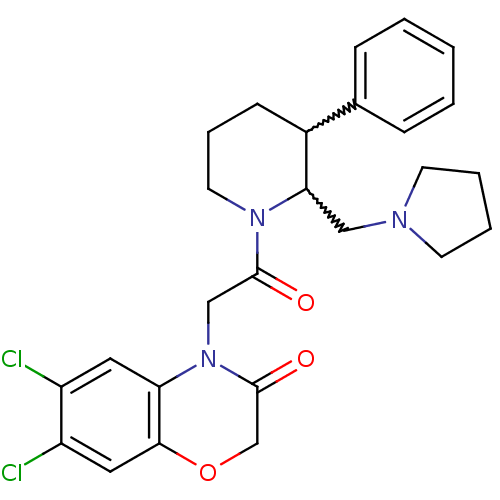
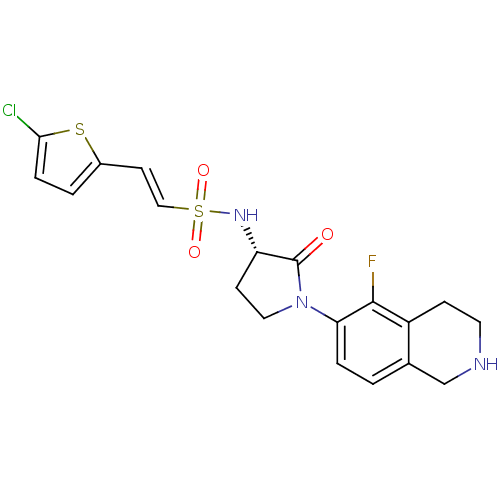
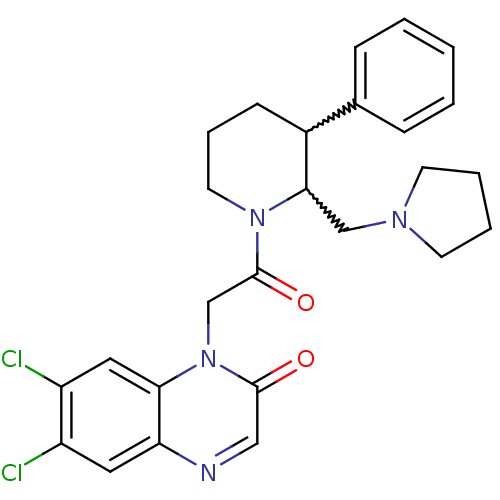
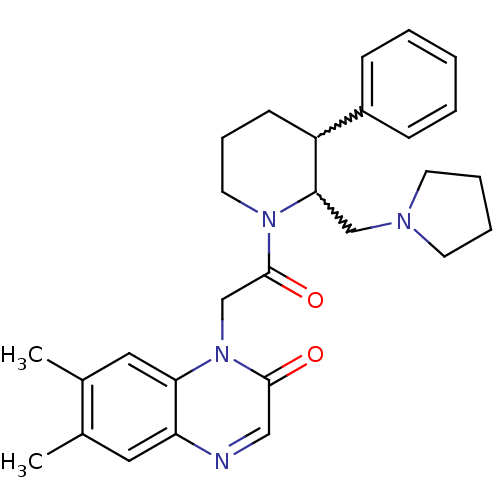
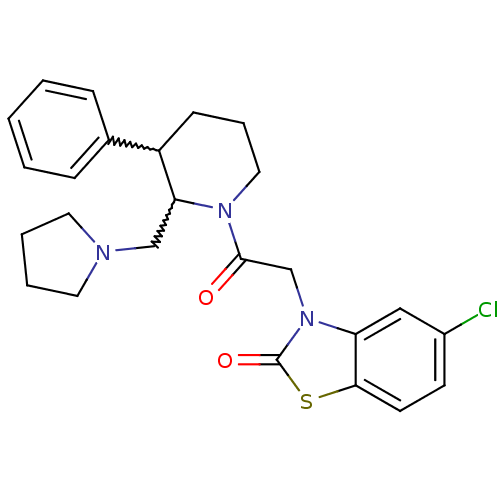
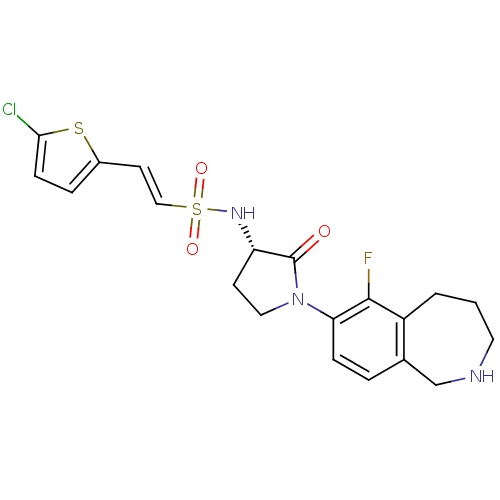
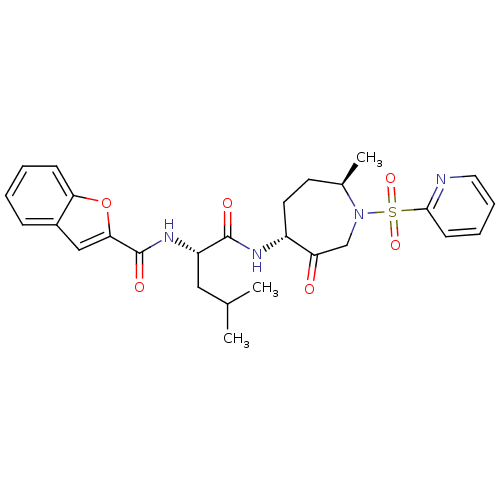
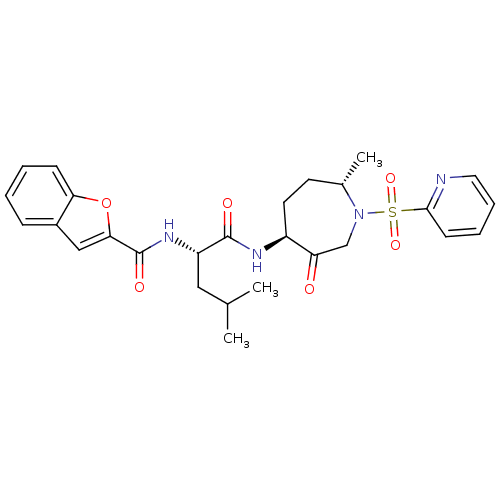
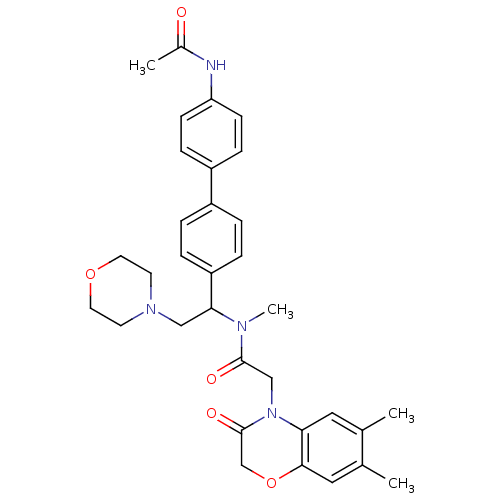
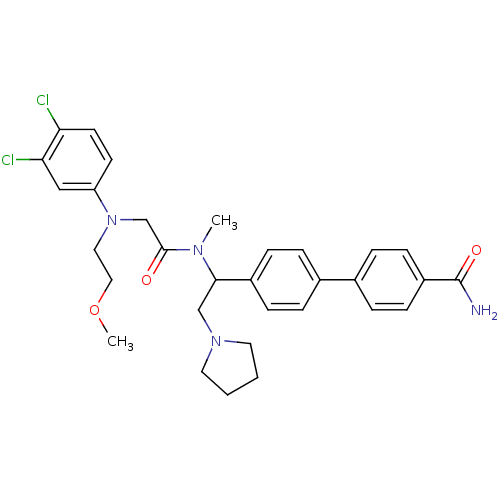
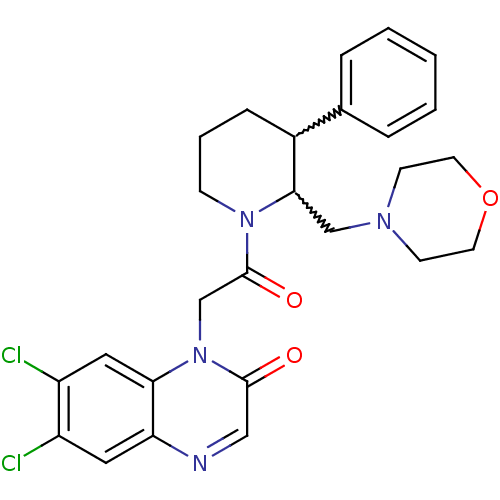
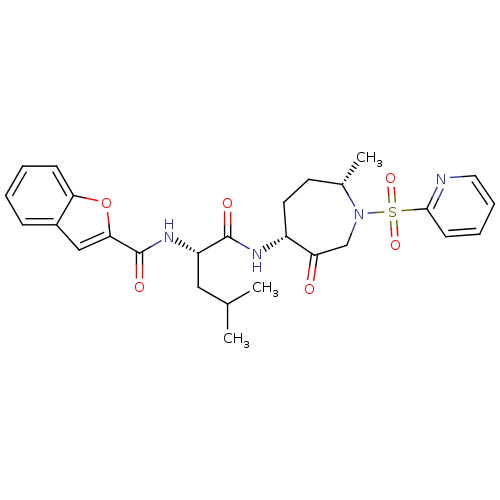
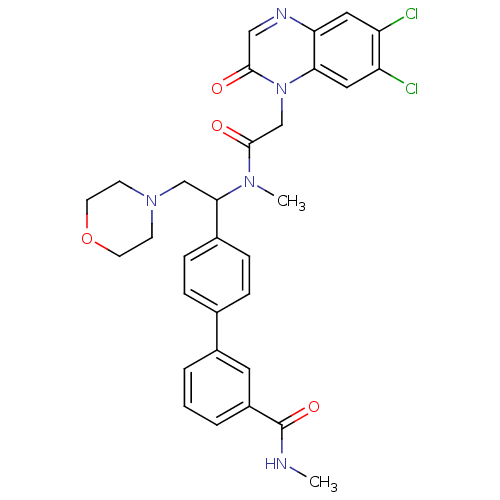
TargetBone morphogenetic protein 1(Homo sapiens (Human))
Glaxosmithkline Pharmaceuticals
Curated by ChEMBL
Glaxosmithkline Pharmaceuticals
Curated by ChEMBL
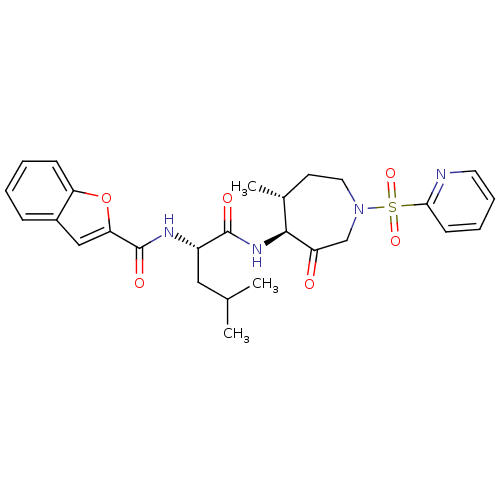
Affinity DataKi: 0.00680nMAssay Description:Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs...More data for this Ligand-Target Pair
Affinity DataKi: 0.00990nM ΔG°: -62.2kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.0310nMAssay Description:Binding affinity to TLL1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst...More data for this Ligand-Target Pair
Affinity DataKi: 0.0390nMAssay Description:Binding affinity to TLL2 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst...More data for this Ligand-Target Pair
Affinity DataKi: 0.0400nM ΔG°: -58.8kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
TargetBone morphogenetic protein 1(Homo sapiens (Human))
Glaxosmithkline Pharmaceuticals
Curated by ChEMBL
Glaxosmithkline Pharmaceuticals
Curated by ChEMBL
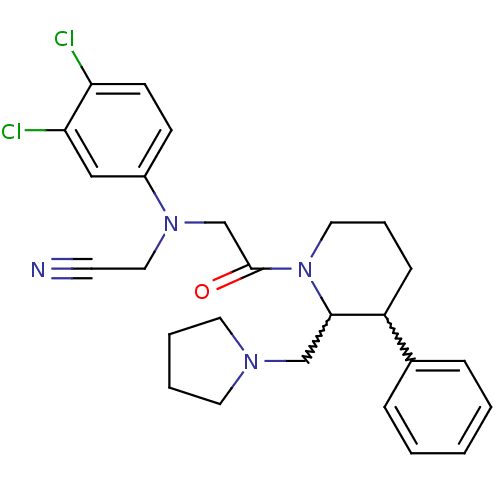
Affinity DataKi: 0.0400nMAssay Description:Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs...More data for this Ligand-Target Pair
Affinity DataKi: 0.0410nM ΔG°: -58.7kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.0630nMAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.0680nM ΔG°: -57.4kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
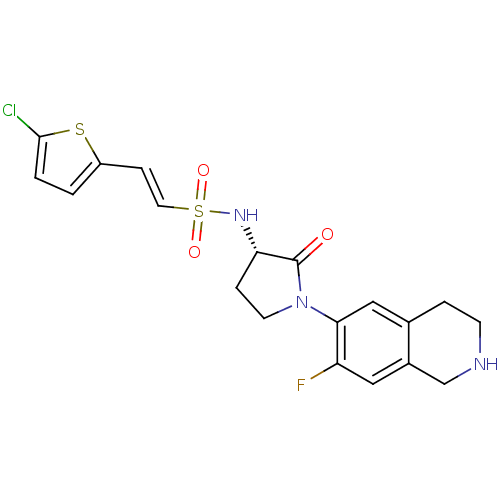
Affinity DataKi: 0.140nM ΔG°: -55.7kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.160nM ΔG°: -55.3kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
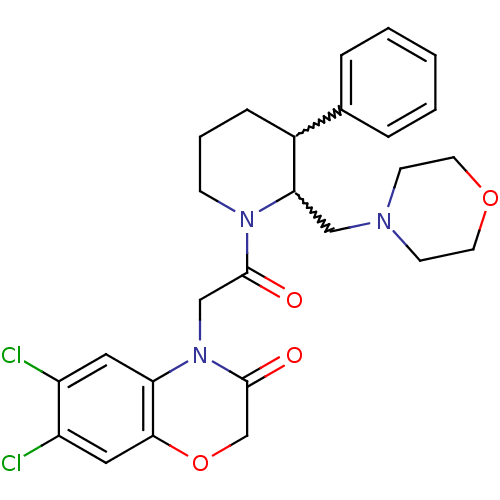
Affinity DataKi: 0.240nMAssay Description:Binding affinity to TLL1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst...More data for this Ligand-Target Pair
Affinity DataKi: 0.260nMAssay Description:Binding affinity to TLL2 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrateMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrateMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.630nM ΔG°: -52.0kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrateMore data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrateMore data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrateMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrateMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrateMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrateMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrateMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrateMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrateMore data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrateMore data for this Ligand-Target Pair
Affinity DataKi: 1.40nM ΔG°: -50.0kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 1.5nM ΔG°: -49.9kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrateMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrateMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrateMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.20nM ΔG°: -48.9kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 2.5nM ΔG°: -48.6kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrateMore data for this Ligand-Target Pair
Affinity DataKi: 3.90nM ΔG°: -47.5kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 4nM ΔG°: -47.5kJ/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)