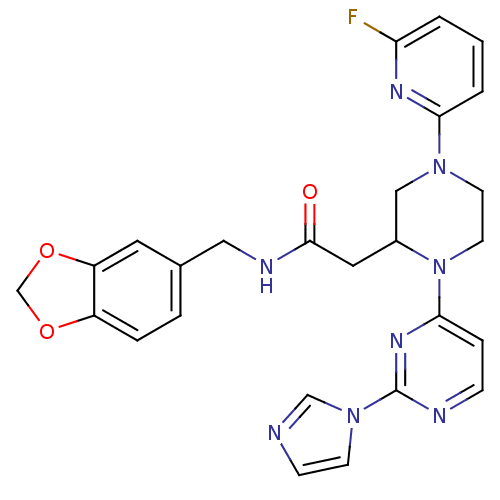
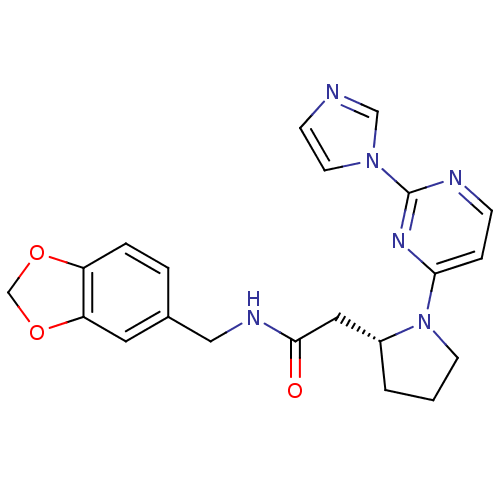
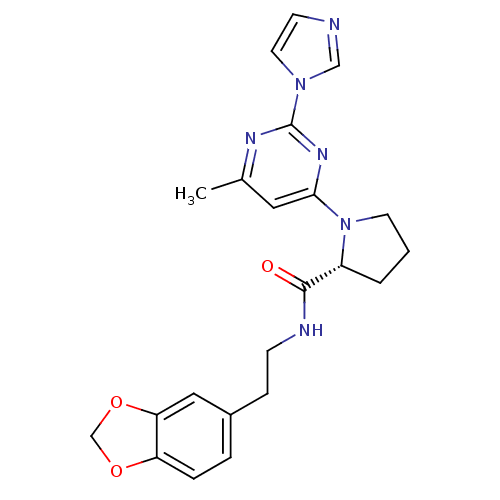
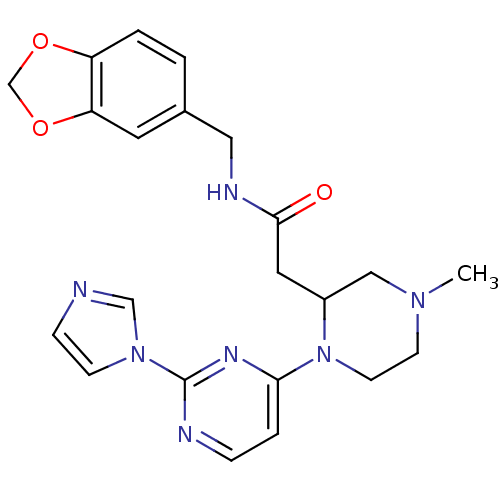
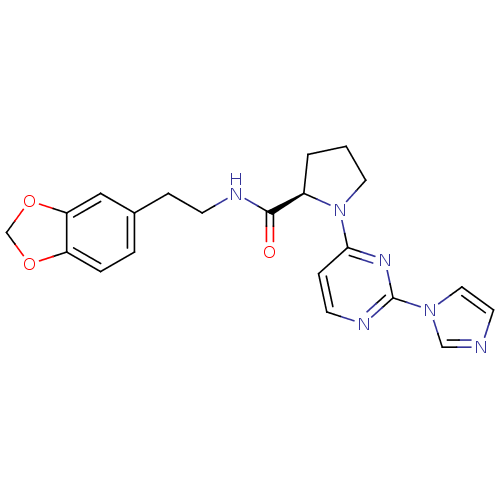
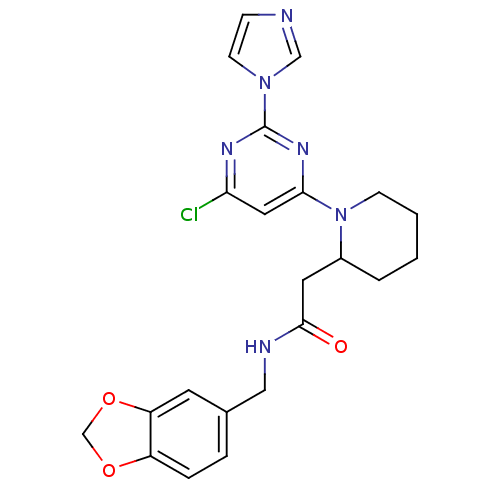
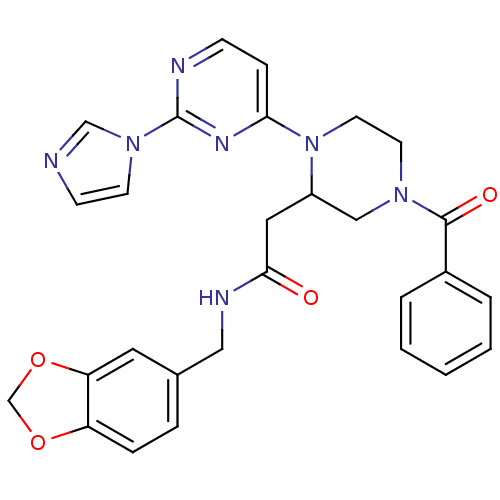
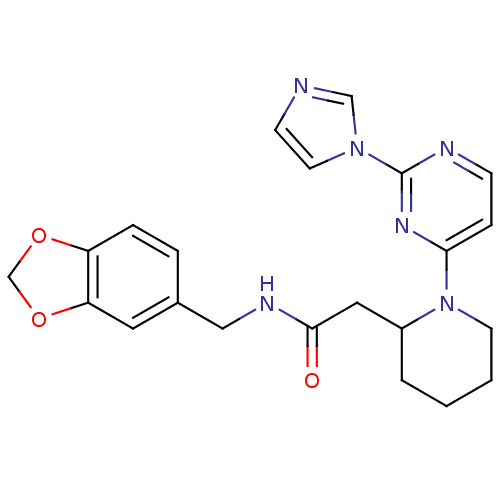
Affinity DataKi: 200nMAssay Description:Competitive inhibition of human recombinant cathepsin L expressed in Pichia pastoris using Z-Phe-Arg-MCA as substrate by Lineweaver-Burk plot analysi...More data for this Ligand-Target Pair
Affinity DataKi: 400nMAssay Description:Competitive inhibition of human recombinant cathepsin V expressed in Pichia pastoris using Z-Phe-Arg-MCA as substrate by Lineweaver-Burk plot analysi...More data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Universidade Federal Fluminense
Curated by ChEMBL
Universidade Federal Fluminense
Curated by ChEMBL
Affinity DataKi: 500nMAssay Description:Inhibition of Human immunodeficiency virus 1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 530nMAssay Description:Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysisMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 780nMAssay Description:Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataKi: 800nMAssay Description:Uncompetitive inhibition of human recombinant cathepsin V expressed in Pichia pastoris using Z-Phe-Arg-MCA as substrate by Lineweaver-Burk plot analy...More data for this Ligand-Target Pair
Affinity DataKi: 800nMAssay Description:Noncompetitive inhibition of human recombinant cathepsin V expressed in Pichia pastoris using Z-Phe-Arg-MCA as substrate by Lineweaver-Burk plot anal...More data for this Ligand-Target Pair
Affinity DataKi: 900nMAssay Description:Competitive inhibition of human recombinant cathepsin L expressed in Pichia pastoris using Z-Phe-Arg-MCA as substrate by Lineweaver-Burk plot analysi...More data for this Ligand-Target Pair
Affinity DataKi: 1.10E+3nMAssay Description:Uncompetitive inhibition of human recombinant cathepsin V expressed in Pichia pastoris using Z-Phe-Arg-MCA as substrate by Lineweaver-Burk plot analy...More data for this Ligand-Target Pair
Affinity DataKi: 1.30E+3nMAssay Description:Competitive inhibition of human recombinant cathepsin V expressed in Pichia pastoris using Z-Phe-Arg-MCA as substrate by Lineweaver-Burk plot analysi...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 1.30E+3nMAssay Description:Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.40E+3nMAssay Description:Mixed-type inhibition of Saccharomyces cerevisiae alpha-glucosidase using PNPG as substrate incubated for 30 mins by Lineweaver-Burk double-reciproca...More data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 1.50E+3nMAssay Description:Noncompetitive inhibition of human recombinant cathepsin L expressed in Pichia pastoris using Z-Phe-Arg-MCA as substrate by Lineweaver-Burk plot anal...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 1.80E+3nMAssay Description:Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.90E+3nMAssay Description:Uncompetitive inhibition of human recombinant cathepsin L expressed in Pichia pastoris using Z-Phe-Arg-MCA as substrate by Lineweaver-Burk plot analy...More data for this Ligand-Target Pair
Affinity DataKi: 1.90E+3nMAssay Description:Uncompetitive inhibition of human recombinant cathepsin L expressed in Pichia pastoris using Z-Phe-Arg-MCA as substrate by Lineweaver-Burk plot analy...More data for this Ligand-Target Pair
Affinity DataKi: 2.80E+3nMAssay Description:Mixed-type inhibition of Saccharomyces cerevisiae alpha-glucosidase using PNPG as substrate incubated for 30 mins by Lineweaver-Burk double-reciproca...More data for this Ligand-Target Pair
Ligand Info
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 3.10E+3nMAssay Description:Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataKi: 7.30E+3nMAssay Description:Mixed-type inhibition of Saccharomyces cerevisiae alpha-glucosidase using PNPG as substrate incubated for 30 mins by Lineweaver-Burk double-reciproca...More data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 8.10E+3nMAssay Description:Mixed-type inhibition of Saccharomyces cerevisiae alpha-glucosidase using PNPG as substrate incubated for 30 mins by Lineweaver-Burk double-reciproca...More data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 8.50E+3nMAssay Description:Mixed-type inhibition of Saccharomyces cerevisiae alpha-glucosidase using PNPG as substrate incubated for 30 mins by Lineweaver-Burk double-reciproca...More data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 1.23E+4nMAssay Description:Mixed-type inhibition of Saccharomyces cerevisiae alpha-glucosidase using PNPG as substrate incubated for 30 mins by Lineweaver-Burk double-reciproca...More data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 1.48E+4nMAssay Description:Mixed-type inhibition of Saccharomyces cerevisiae alpha-glucosidase using PNPG as substrate incubated for 30 mins by Lineweaver-Burk double-reciproca...More data for this Ligand-Target Pair
Ligand Info
TargetGlyceraldehyde-3-phosphate dehydrogenase, glycosomal(Trypanosoma cruzi)
Da Universidade De S£O Paulo
Curated by ChEMBL
Da Universidade De S£O Paulo
Curated by ChEMBL
Affinity DataKi: 1.60E+4nMAssay Description:Competitive inhibition of Trypanosoma cruzi glycosomal GAPDH NAD+ binding site by ITC methodMore data for this Ligand-Target Pair
Affinity DataKi: 1.70E+4nMAssay Description:Non-competitive inhibition of Leishmania amazonensis arginase using L-arginine as substrate incubated for 15 minsMore data for this Ligand-Target Pair
TargetGlyceraldehyde-3-phosphate dehydrogenase, glycosomal(Trypanosoma cruzi)
Da Universidade De S£O Paulo
Curated by ChEMBL
Da Universidade De S£O Paulo
Curated by ChEMBL
Affinity DataKi: 1.71E+4nMAssay Description:Competitive inhibition of Trypanosoma cruzi glycosomal GAPDH NAD+ binding site by ITC methodMore data for this Ligand-Target Pair
Affinity DataKi: 1.81E+4nMAssay Description:Mixed-type inhibition of Saccharomyces cerevisiae alpha-glucosidase using PNPG as substrate incubated for 30 mins by Lineweaver-Burk double-reciproca...More data for this Ligand-Target Pair
TargetGlyceraldehyde-3-phosphate dehydrogenase, glycosomal(Trypanosoma cruzi)
Da Universidade De S£O Paulo
Curated by ChEMBL
Da Universidade De S£O Paulo
Curated by ChEMBL
Affinity DataKi: 1.87E+4nMAssay Description:Competitive inhibition of Trypanosoma cruzi glycosomal GAPDH NAD+ binding site by ITC methodMore data for this Ligand-Target Pair
Affinity DataKi: 2.30E+4nMAssay Description:Mixed-type inhibition of Saccharomyces cerevisiae alpha-glucosidase using PNPG as substrate incubated for 30 mins by Lineweaver-Burk double-reciproca...More data for this Ligand-Target Pair
Ligand Info
TargetGlyceraldehyde-3-phosphate dehydrogenase, glycosomal(Trypanosoma cruzi)
Da Universidade De S£O Paulo
Curated by ChEMBL
Da Universidade De S£O Paulo
Curated by ChEMBL
Affinity DataKi: 2.95E+4nMAssay Description:Competitive inhibition of Trypanosoma cruzi glycosomal GAPDH NAD+ binding site by ITC methodMore data for this Ligand-Target Pair
TargetGlyceraldehyde-3-phosphate dehydrogenase, glycosomal(Trypanosoma cruzi)
Da Universidade De S£O Paulo
Curated by ChEMBL
Da Universidade De S£O Paulo
Curated by ChEMBL
Affinity DataKi: 3.23E+4nMAssay Description:Competitive inhibition of Trypanosoma cruzi glycosomal GAPDH NAD+ binding site by ITC methodMore data for this Ligand-Target Pair
TargetGlyceraldehyde-3-phosphate dehydrogenase, glycosomal(Trypanosoma cruzi)
Da Universidade De S£O Paulo
Curated by ChEMBL
Da Universidade De S£O Paulo
Curated by ChEMBL
Affinity DataKi: 5.76E+4nMAssay Description:Competitive inhibition of Trypanosoma cruzi glycosomal GAPDH NAD+ binding site by ITC methodMore data for this Ligand-Target Pair
TargetGlyceraldehyde-3-phosphate dehydrogenase, glycosomal(Trypanosoma cruzi)
Da Universidade De S£O Paulo
Curated by ChEMBL
Da Universidade De S£O Paulo
Curated by ChEMBL
Affinity DataKi: 5.95E+4nMAssay Description:Competitive inhibition of Trypanosoma cruzi glycosomal GAPDH NAD+ binding site by ITC methodMore data for this Ligand-Target Pair
TargetGlyceraldehyde-3-phosphate dehydrogenase, glycosomal(Trypanosoma cruzi)
Da Universidade De S£O Paulo
Curated by ChEMBL
Da Universidade De S£O Paulo
Curated by ChEMBL
Affinity DataKi: 1.05E+5nMAssay Description:Non-competitive inhibition of Trypanosoma cruzi glycosomal GAPDH active site by ITC methodMore data for this Ligand-Target Pair
TargetGlyceraldehyde-3-phosphate dehydrogenase, glycosomal(Trypanosoma cruzi)
Da Universidade De S£O Paulo
Curated by ChEMBL
Da Universidade De S£O Paulo
Curated by ChEMBL
Affinity DataKi: 1.51E+5nMAssay Description:Non-competitive inhibition of Trypanosoma cruzi glycosomal GAPDH active site by ITC methodMore data for this Ligand-Target Pair
TargetGlyceraldehyde-3-phosphate dehydrogenase, glycosomal(Trypanosoma cruzi)
Da Universidade De S£O Paulo
Curated by ChEMBL
Da Universidade De S£O Paulo
Curated by ChEMBL
Affinity DataKi: 3.05E+5nMAssay Description:Non-competitive inhibition of Trypanosoma cruzi glycosomal GAPDH active site by ITC methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.120nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.130nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
Affinity DataIC50: 0.240nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.280nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.280nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.290nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.380nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.480nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.480nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.490nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formationMore data for this Ligand-Target Pair


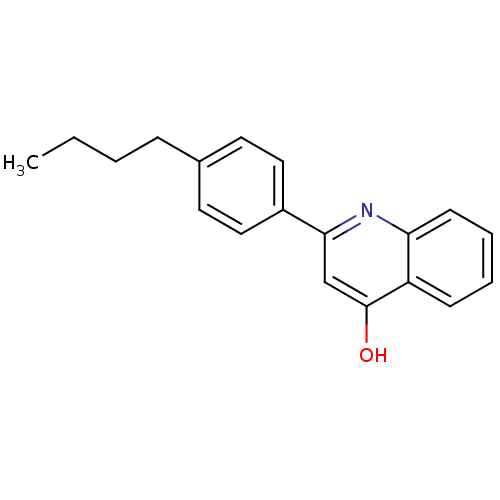
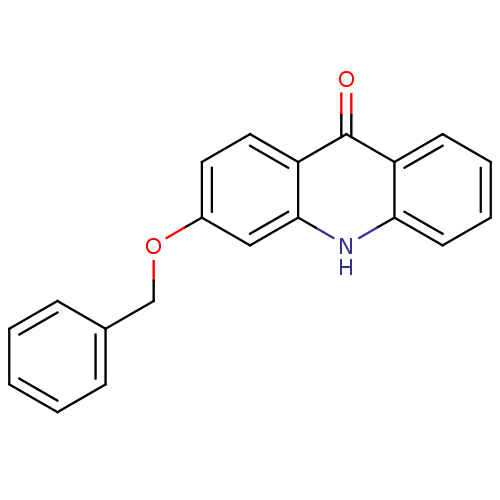
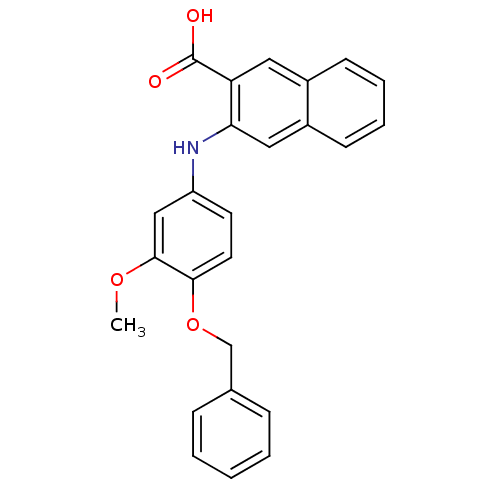
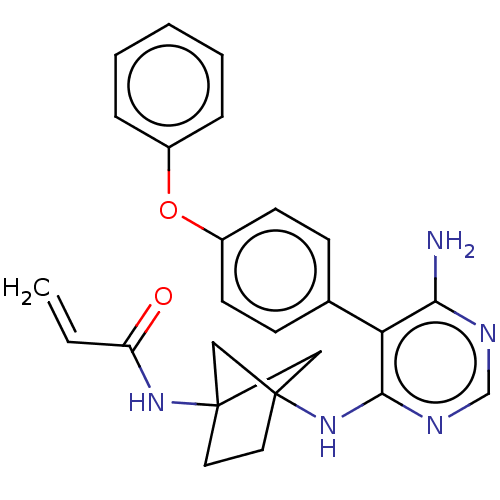
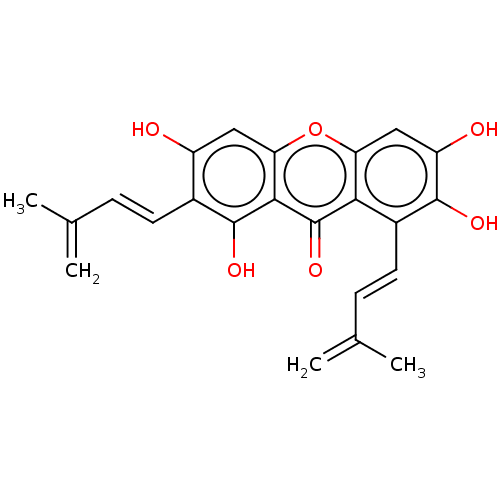
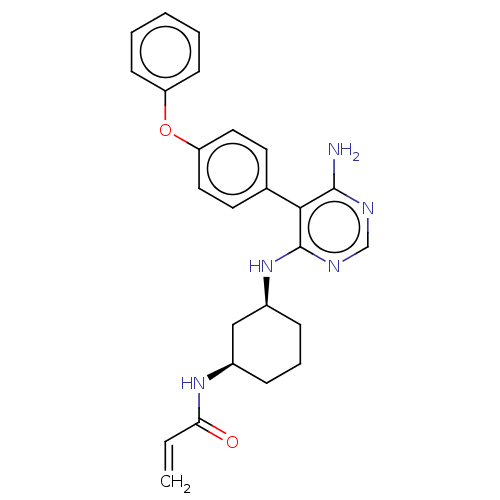
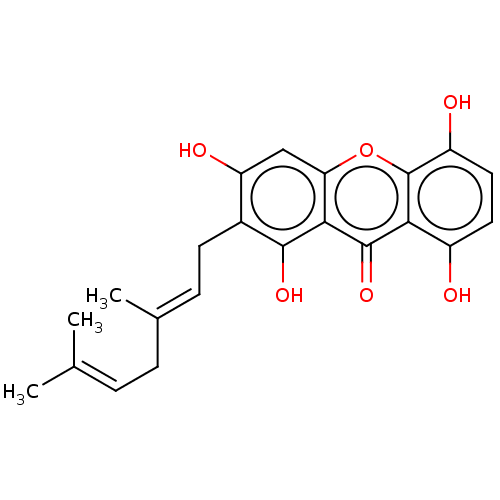
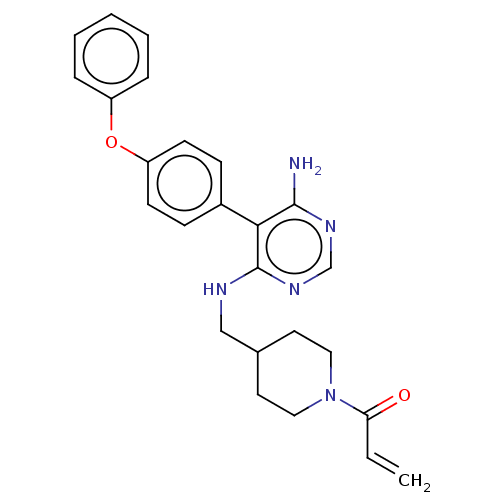

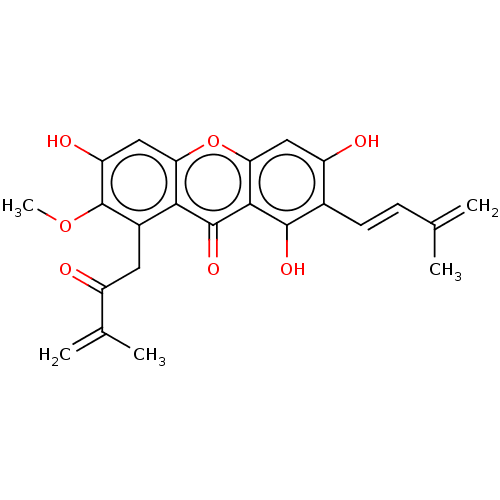
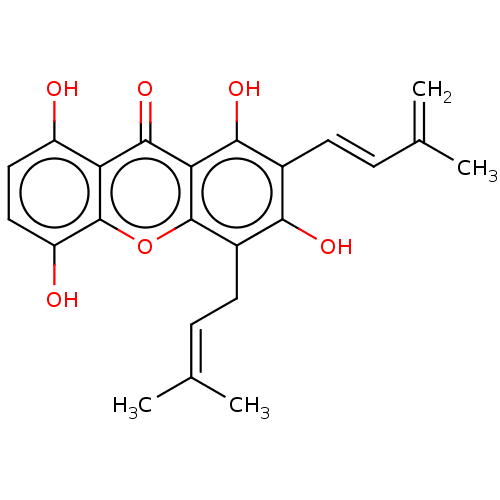

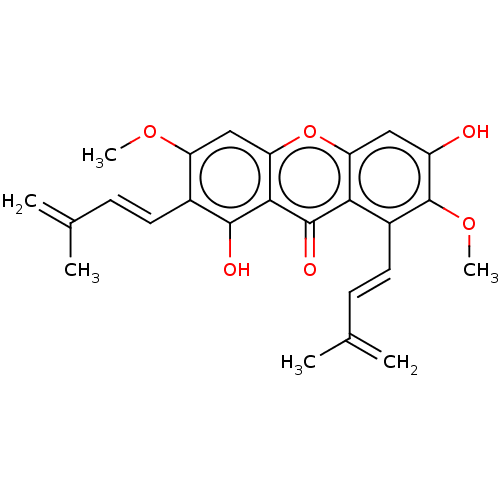

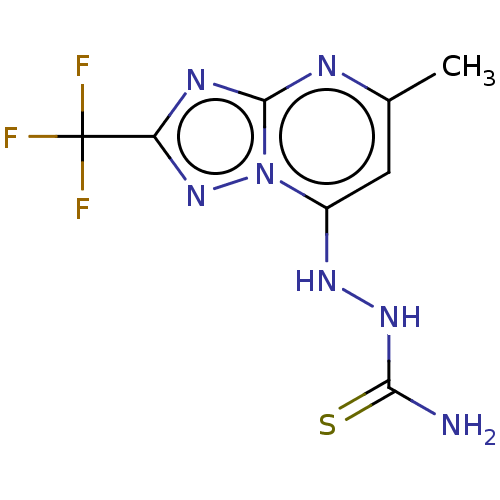
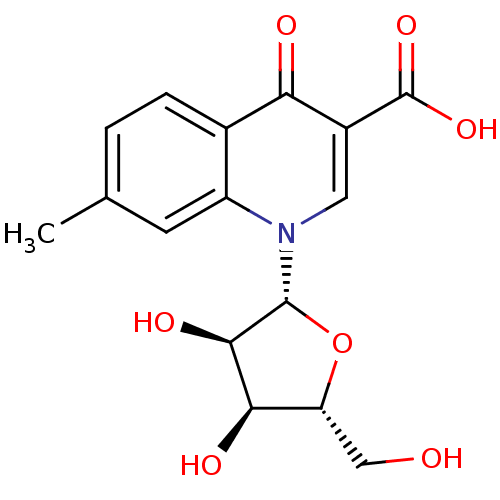

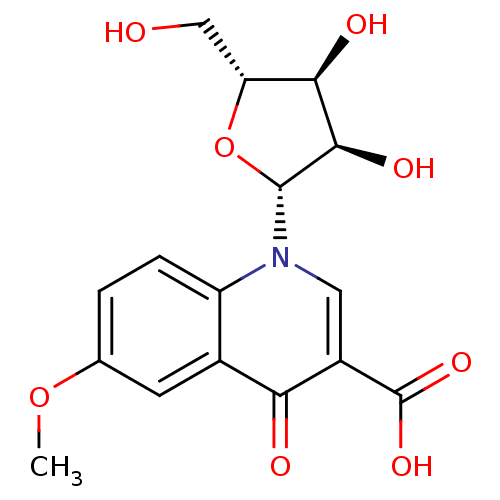
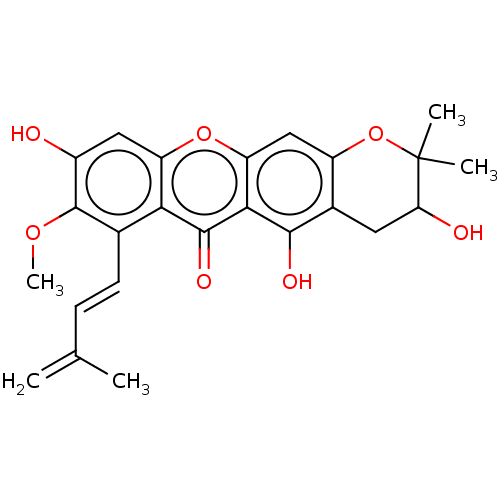

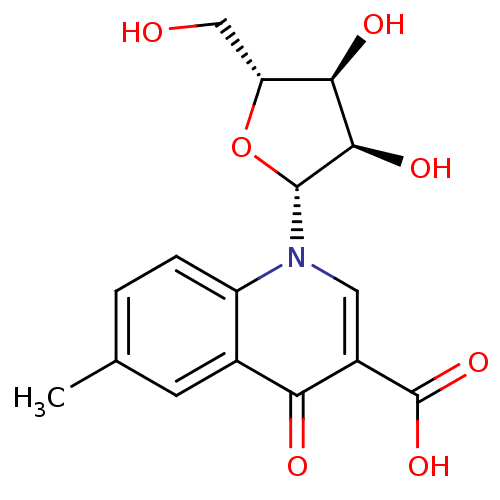
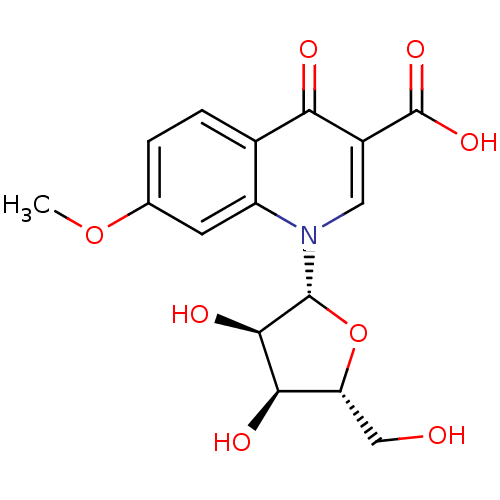
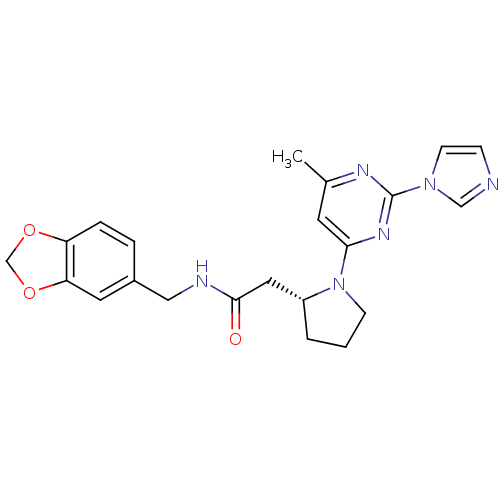
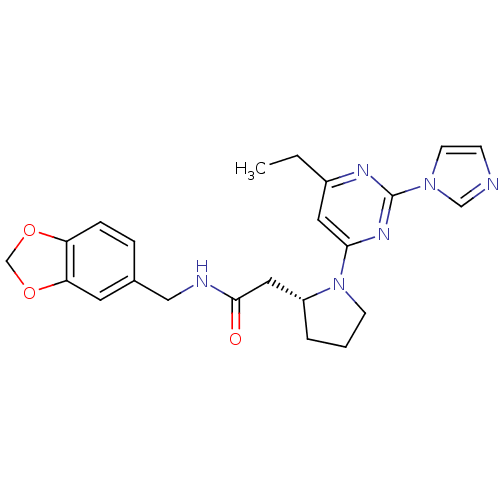
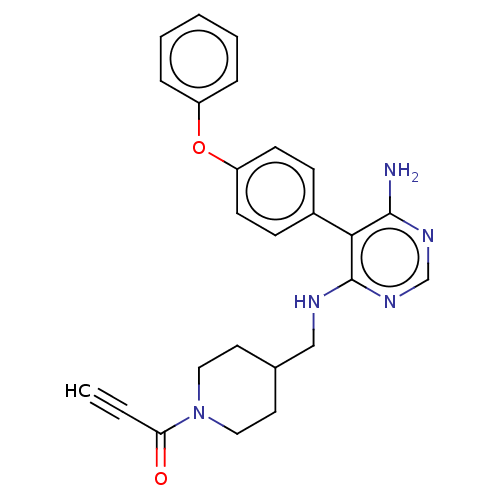
 3D Structure (crystal)
3D Structure (crystal)