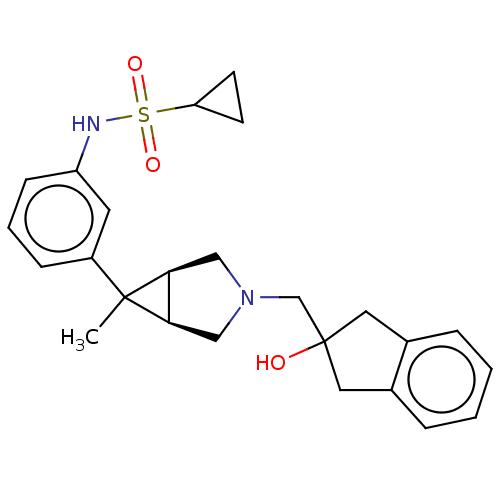
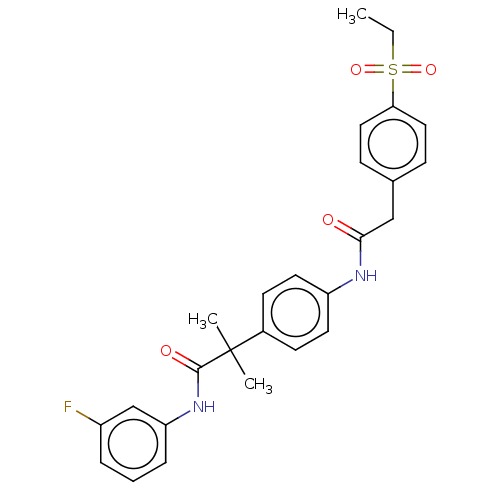
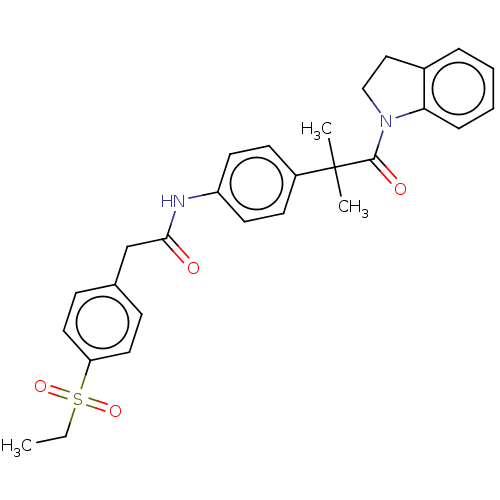
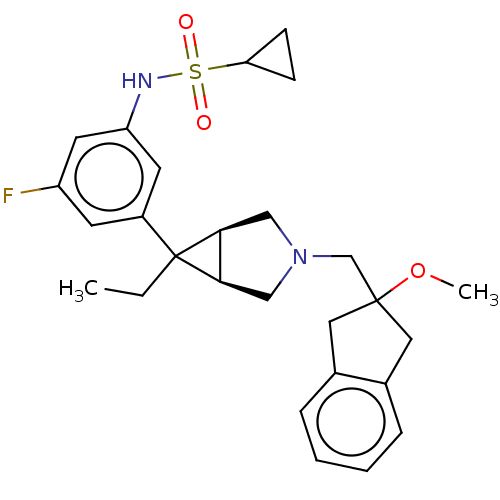
Affinity DataKi: 370nMAssay Description:Compound was tested in vitro for the inhibition of the specific binding of [3H]-Gly to N-methyl-D-aspartate glutamate receptor 1 in rat whole brain m...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, kainate 1(RAT)
Yamanouchi Pharmaceutical
Curated by PDSP Ki Database
Yamanouchi Pharmaceutical
Curated by PDSP Ki Database
TargetGlutamate receptor ionotropic, kainate 1(RAT)
Yamanouchi Pharmaceutical
Curated by PDSP Ki Database
Yamanouchi Pharmaceutical
Curated by PDSP Ki Database
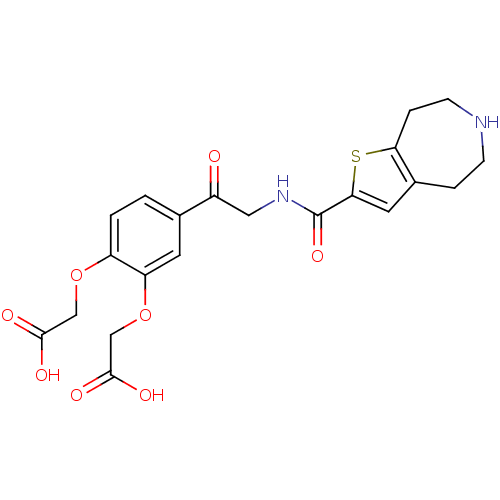
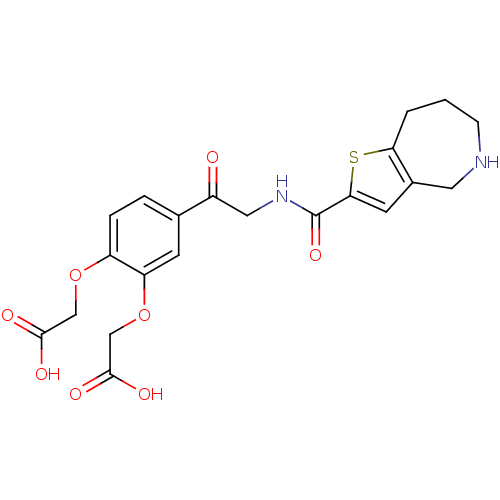
Affinity DataKi: 2.30E+3nMAssay Description:Inhibition of human recombinant CES2 assessed as compound hydrolysisMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, kainate 1(RAT)
Yamanouchi Pharmaceutical
Curated by PDSP Ki Database
Yamanouchi Pharmaceutical
Curated by PDSP Ki Database
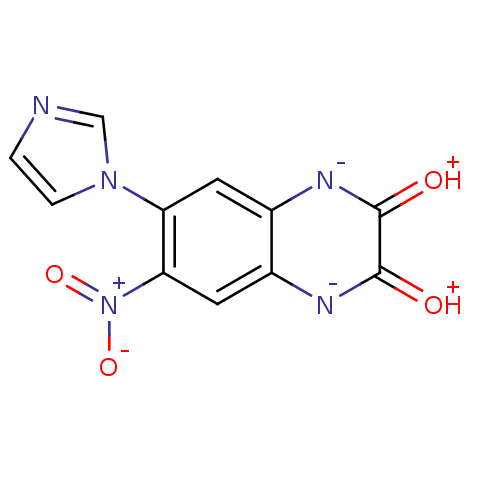
Affinity DataKi: 4.20E+3nMAssay Description:Binding affinity of the compound towards glycine binding site on NMDA receptor was determined in rat whole brain membrane using strychnine-insensitiv...More data for this Ligand-Target Pair
Affinity DataKi: 8.00E+3nMAssay Description:Compound was tested in vitro for the inhibition of the specific binding of [3H]-Gly to N-methyl-D-aspartate glutamate receptor 1 in rat whole brain m...More data for this Ligand-Target Pair
Affinity DataKi: 3.70E+4nMAssay Description:Binding affinity of the compound towards glycine binding site on NMDA receptor was determined in rat whole brain membrane using strychnine-insensitiv...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity of the compound towards glycine binding site on NMDA receptor was determined in rat whole brain membrane using strychnine-insensitiv...More data for this Ligand-Target Pair
TargetParathyroid hormone/parathyroid hormone-related peptide receptor(Homo sapiens (Human))
Chugai Pharmaceutical
Curated by ChEMBL
Chugai Pharmaceutical
Curated by ChEMBL
Affinity DataIC50: 0.0800nMAssay Description:Displacement of 125I-PTH (1 to 15 residues) from human PTHR1 expressed in African green monkey COS7 cell membranes at 300 uM after 90 mins by gamma c...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of Vitronectin binding to GPIIb/IIIIa Vitronectin receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 2.90nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Antagonist activity at VDR expressed in COS7 cells assessed as inhibition of 1,25-Dihydroxyvitamin D3-induced response by transient transcription ass...More data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 3.60nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 4.10nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 4.10nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 4.40nMAssay Description:Transrepression of recombinant human GAL4-DBD fused RORgammat LBD expressed in HEK293 cells after 20 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 4.70nMAssay Description:Biotinylated fibrinogen binding to alpha IIb beta3 integrinMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70nMAssay Description:Inhibition of biotinylated fibrinogen binding to alphaIIb-beta3 integrinMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of ACE by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.40nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of vWF binding to alphaIIb-beta3 integrinMore data for this Ligand-Target Pair
Affinity DataIC50: 8.20nMAssay Description:Transrepression of recombinant human GAL4-DBD fused RORgammat LBD expressed in HEK293 cells after 20 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 8.70nMAssay Description:Inhibition of biotinylated fibrinogen binding to alphaIIb-beta3 integrinMore data for this Ligand-Target Pair
Affinity DataIC50: 9.80nMAssay Description:Transrepression of recombinant human GAL4-DBD fused RORgammat LBD expressed in HEK293 cells after 20 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 9.80nMAssay Description:Biotinylated fibrinogen binding to alpha IIb beta3 integrinMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Transrepression of recombinant human GAL4-DBD fused RORgammat LBD expressed in HEK293 cells after 20 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO...More data for this Ligand-Target Pair


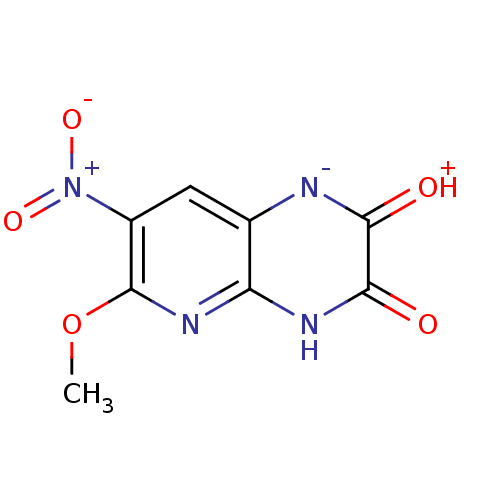

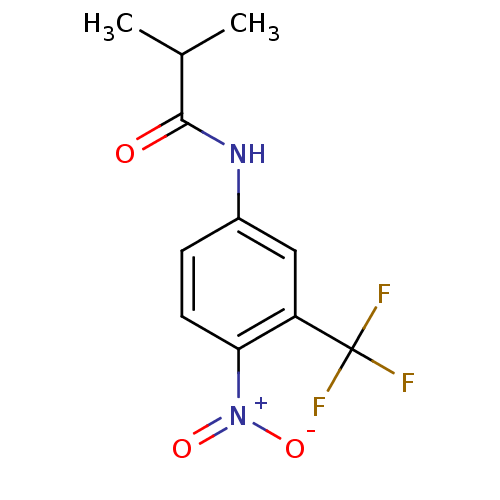
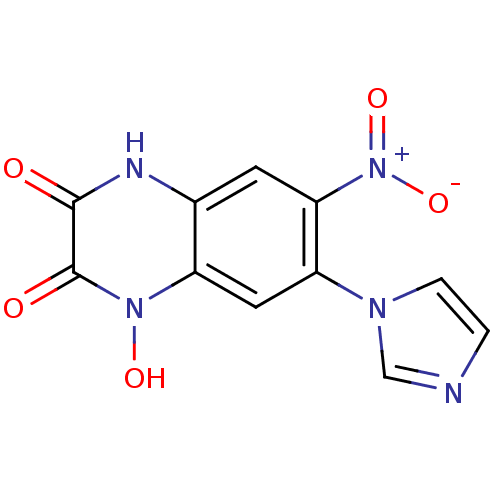
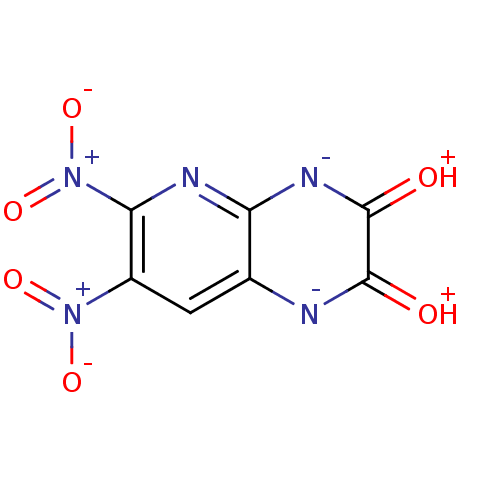



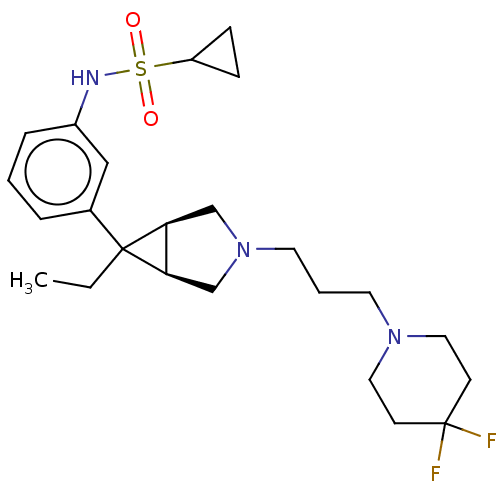

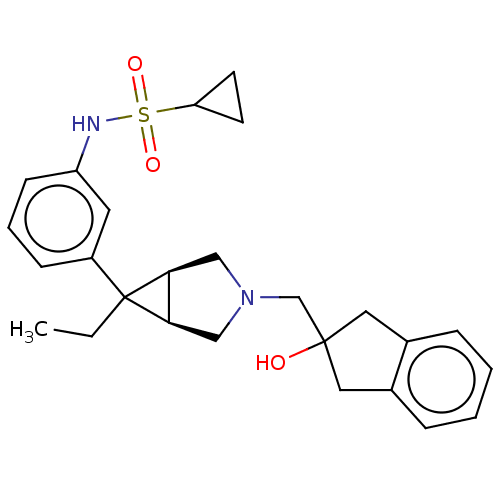
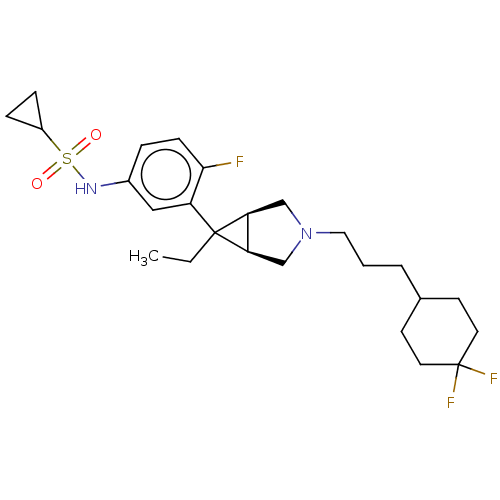
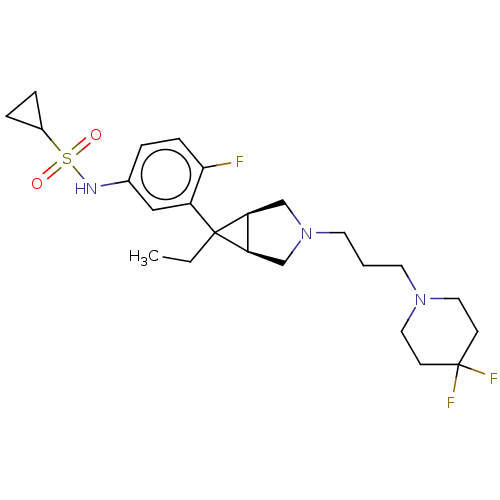
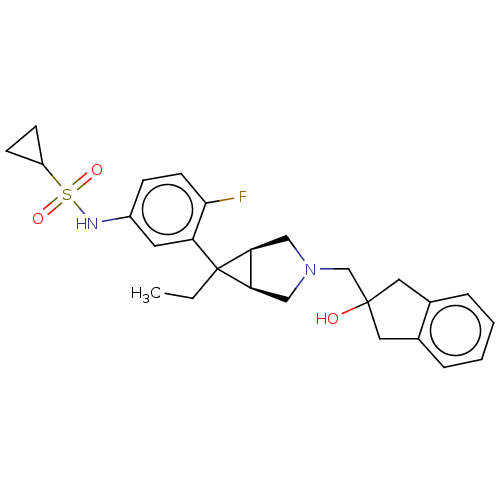

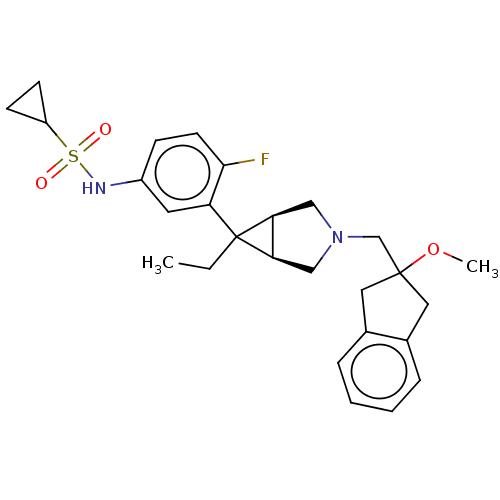
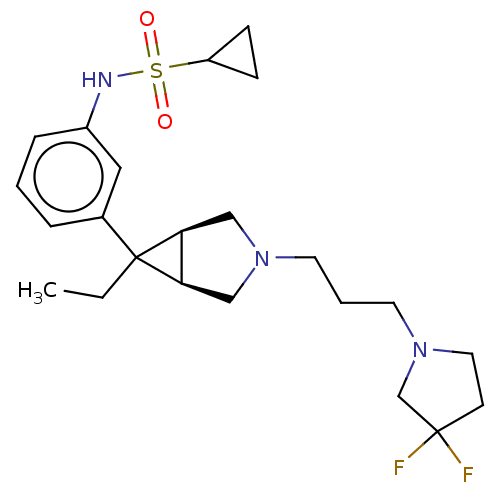
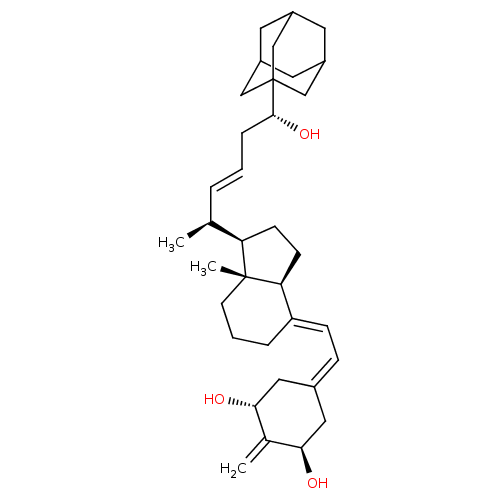
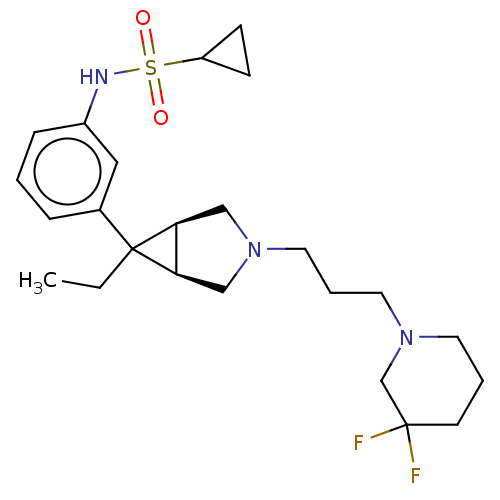
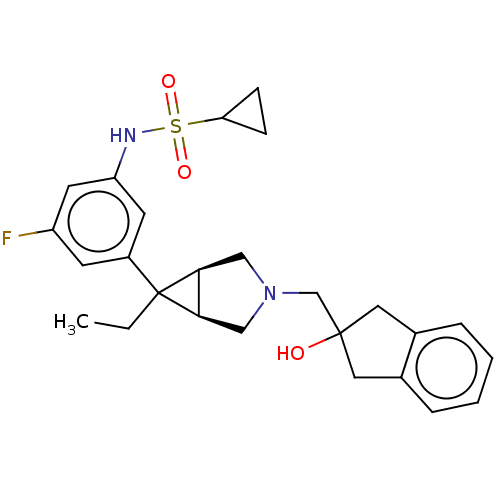
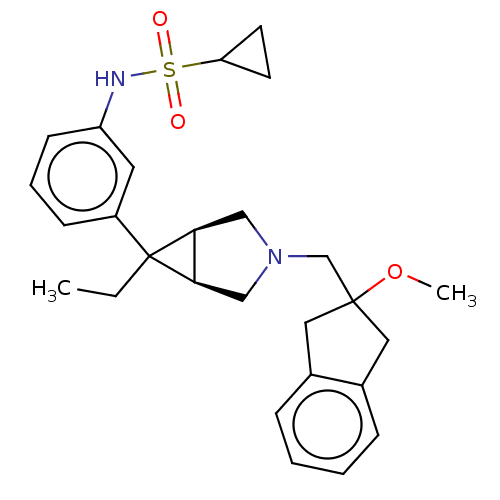
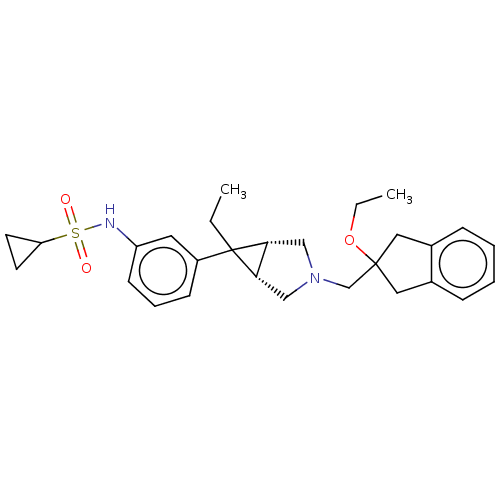



 3D Structure (crystal)
3D Structure (crystal)