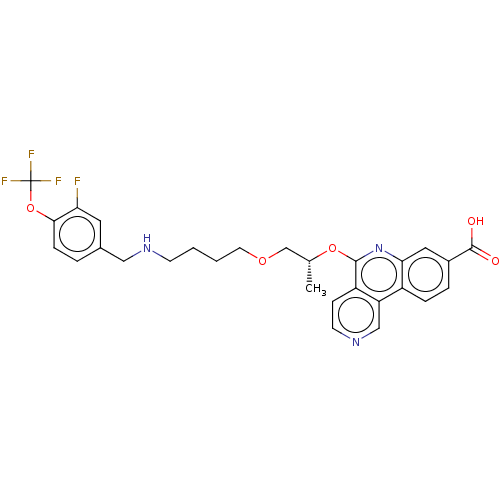
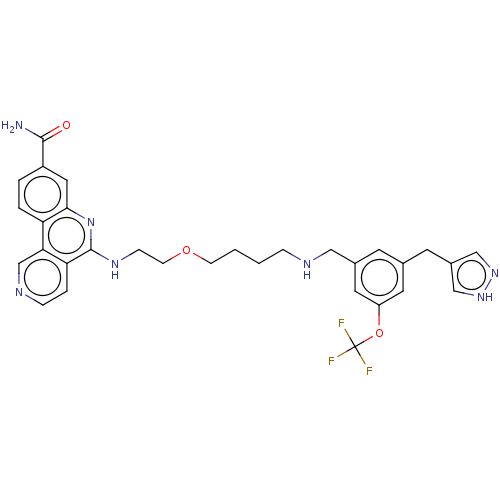
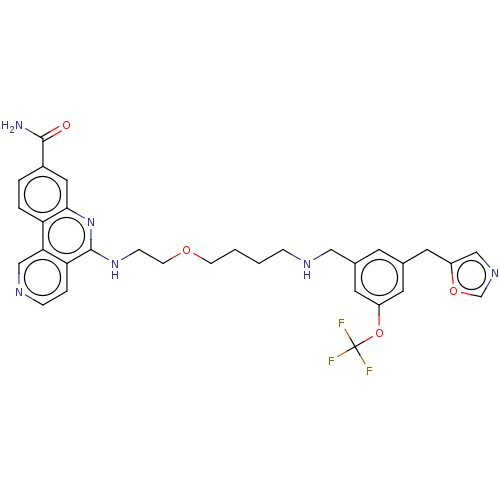
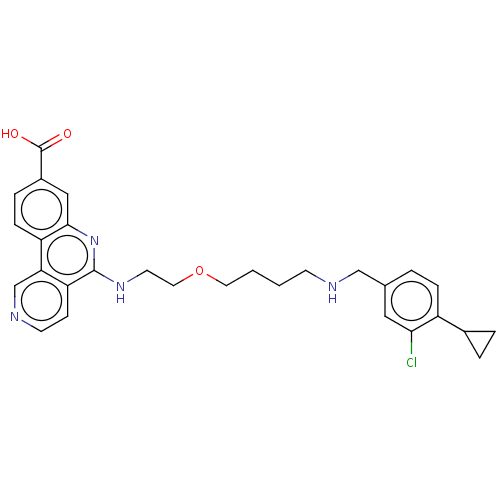
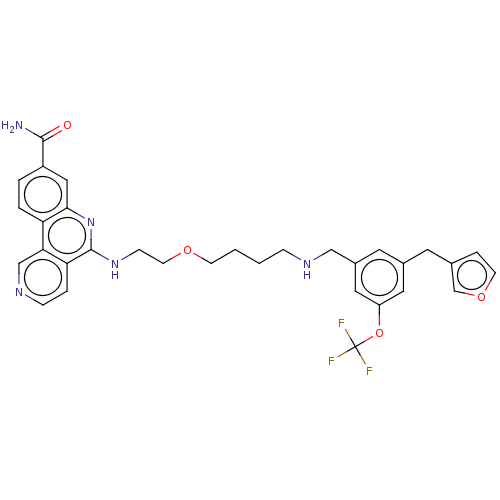
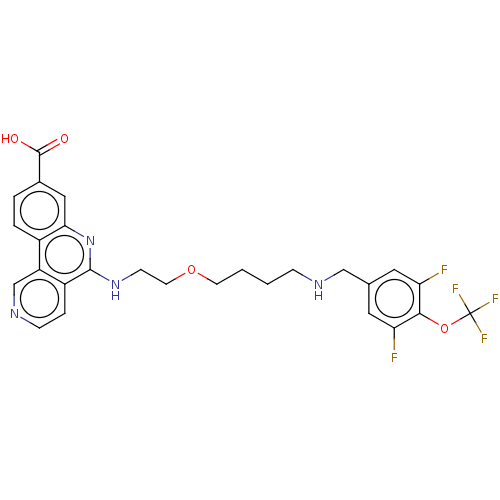
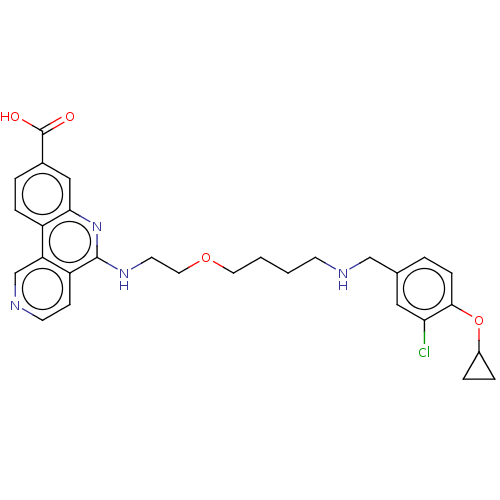
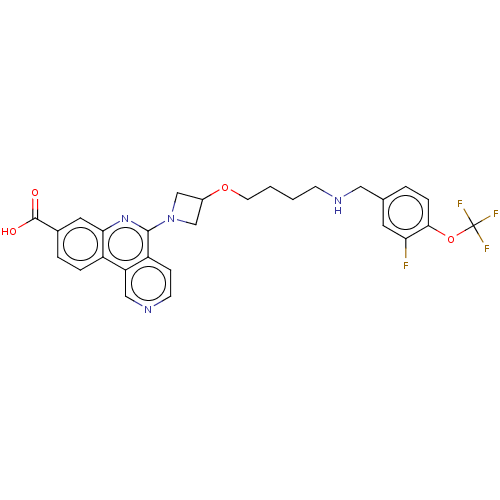
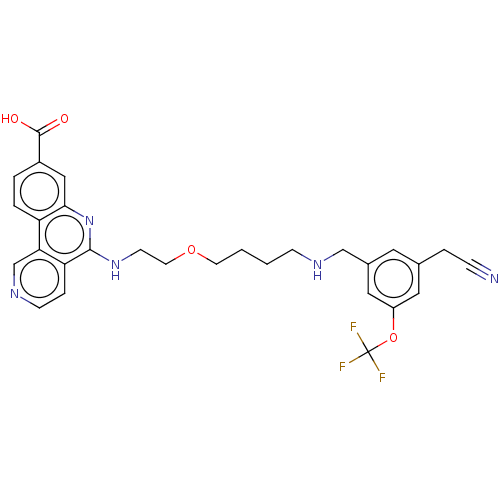
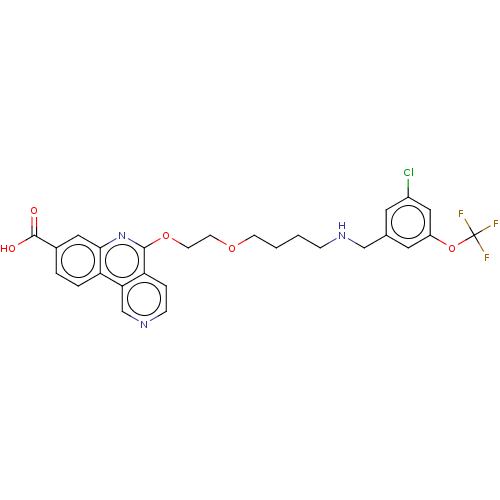
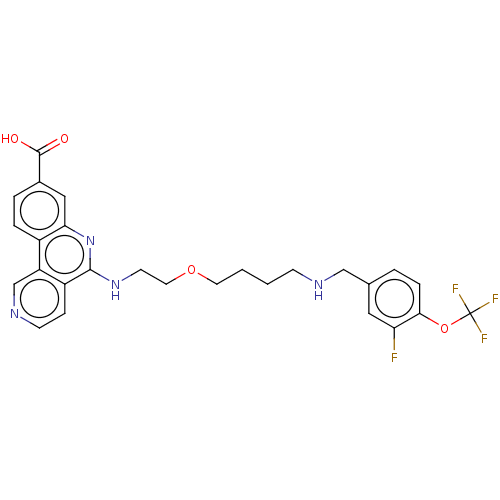
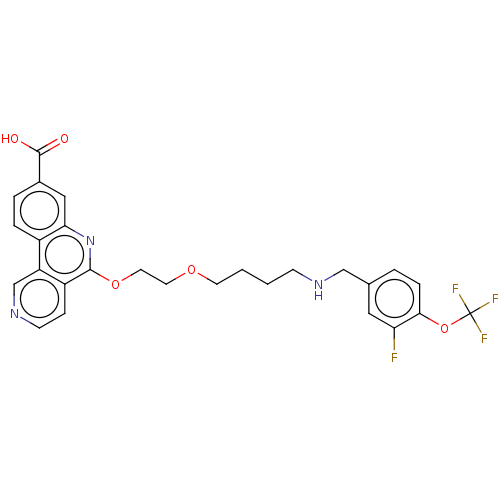
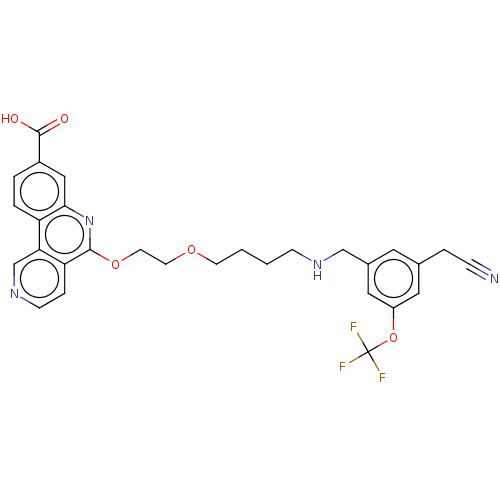
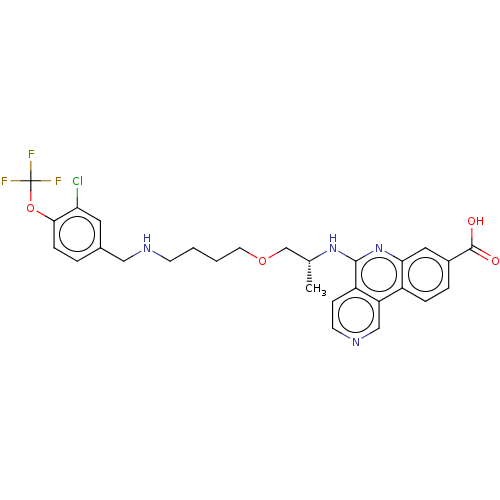
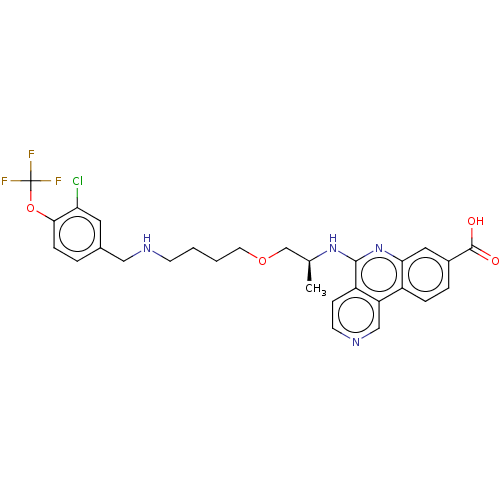
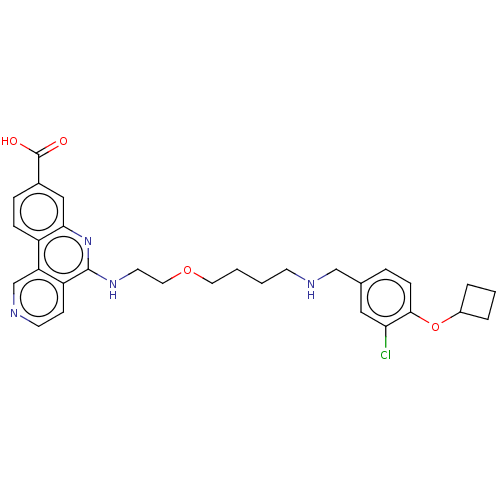
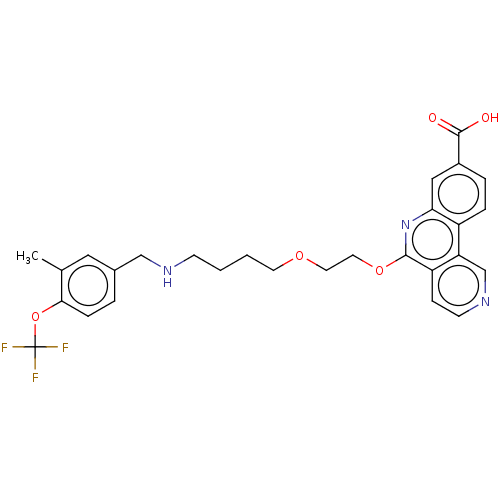
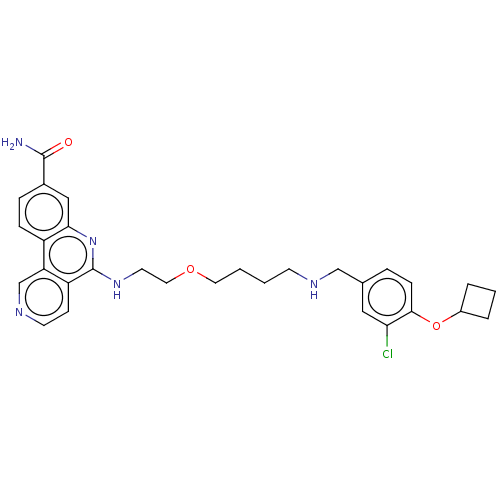
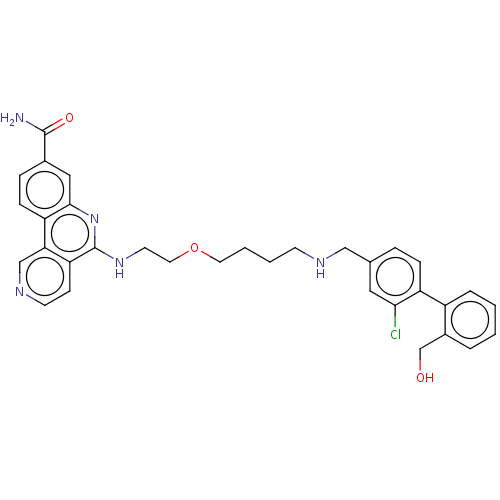
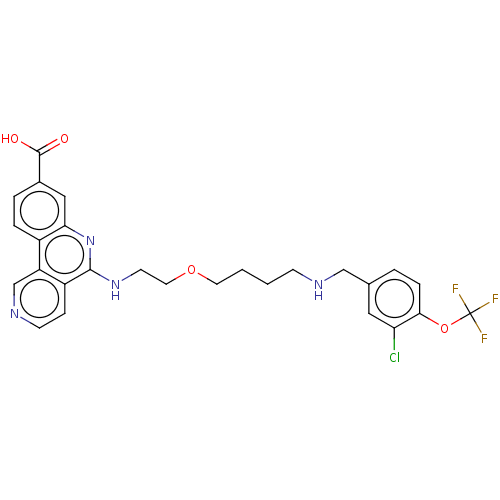
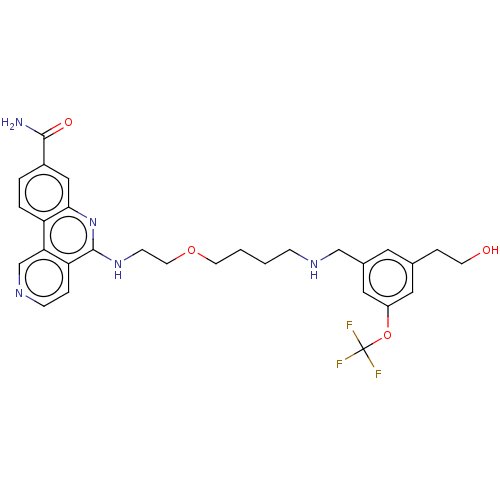
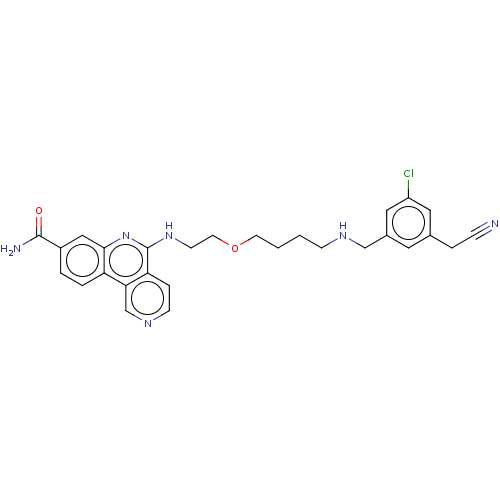
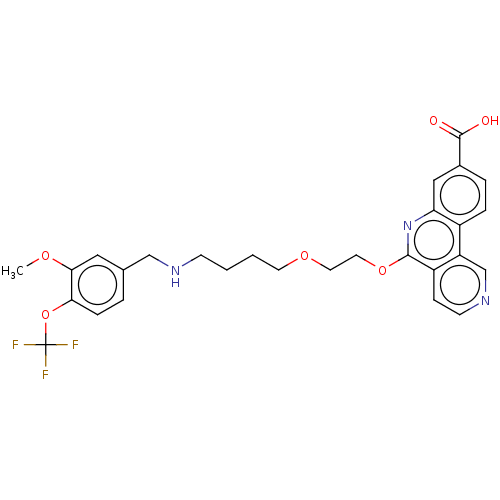
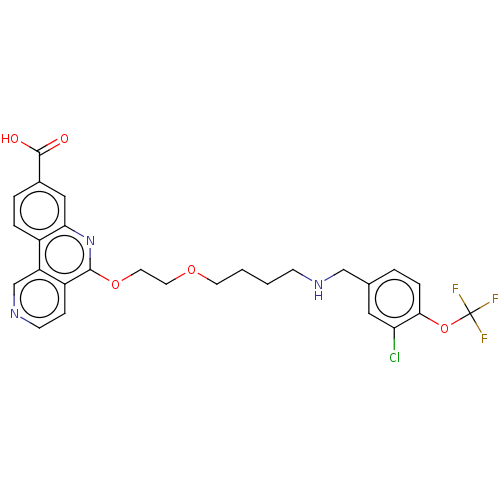
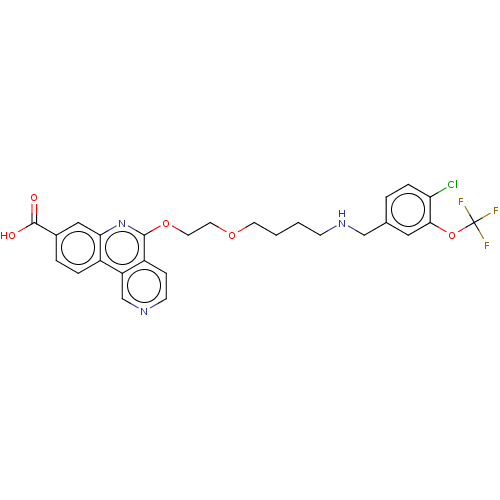
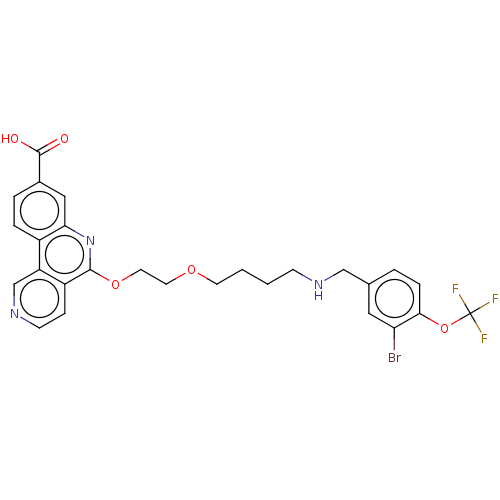
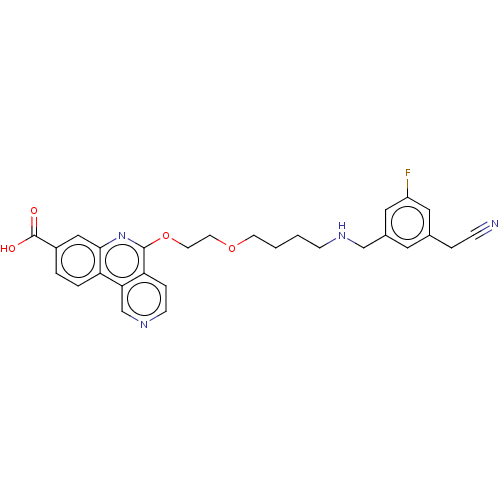
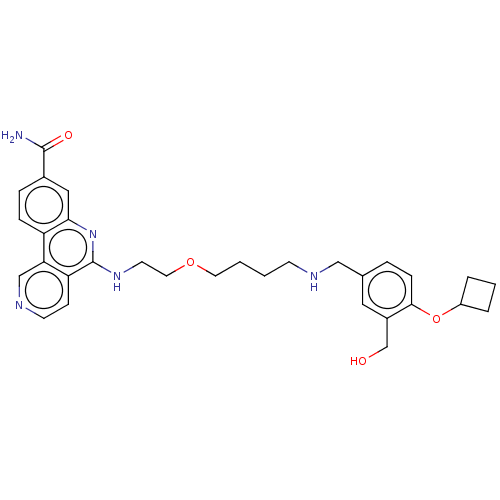
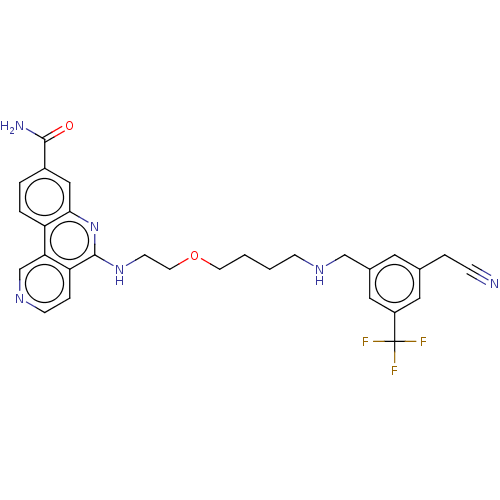
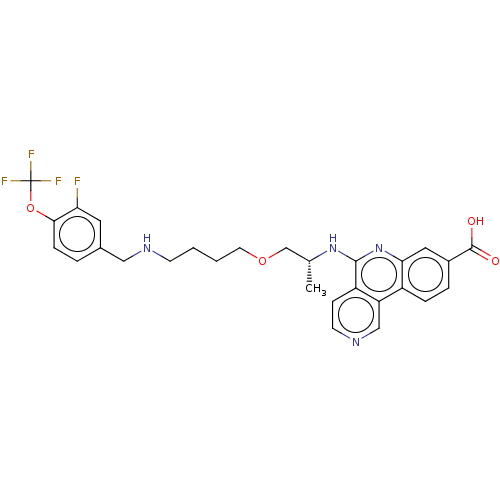
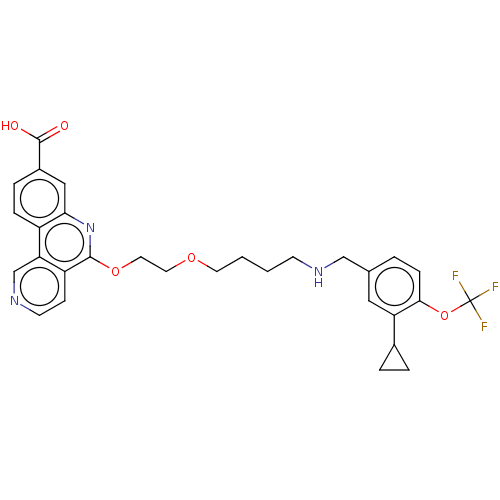
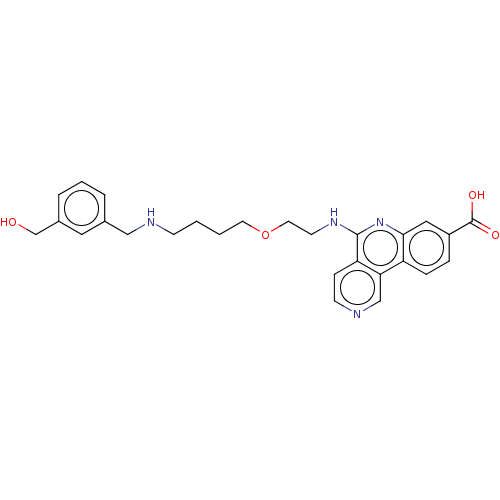
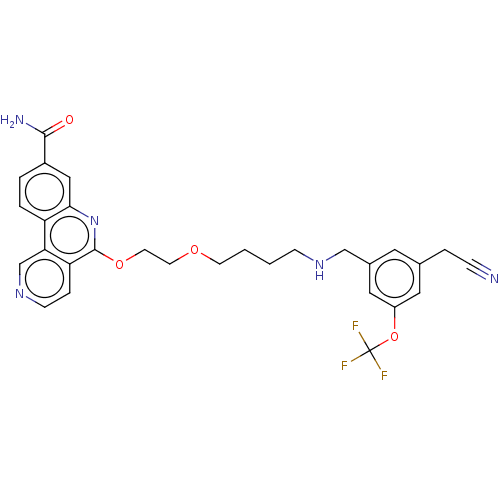
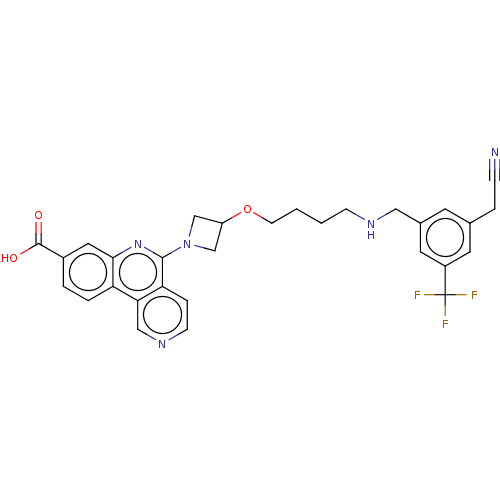
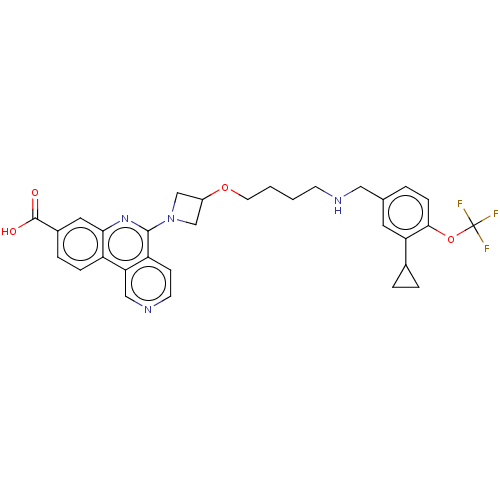
Affinity DataIC50: 0.145nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.180nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.197nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.201nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.203nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.217nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.221nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.223nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.236nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.244nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.248nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.248nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.258nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.263nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.265nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.278nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.298nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.304nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.306nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.318nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.324nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.333nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.342nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.346nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.349nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.354nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.354nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.359nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.361nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.365nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.366nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.370nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.372nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.378nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.382nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.390nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.403nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.403nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.404nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.409nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.411nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.418nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.425nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.427nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.428nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.432nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.436nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.437nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.453nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair
Affinity DataIC50: 0.457nMAssay Description:Final assay conditions comprised 0.2 nM CK2α, 50 μM peptide substrate (RRRADDSDDDDD), 15 μM ATP in 1× reaction buffer (40 mM Tris pH7....More data for this Ligand-Target Pair