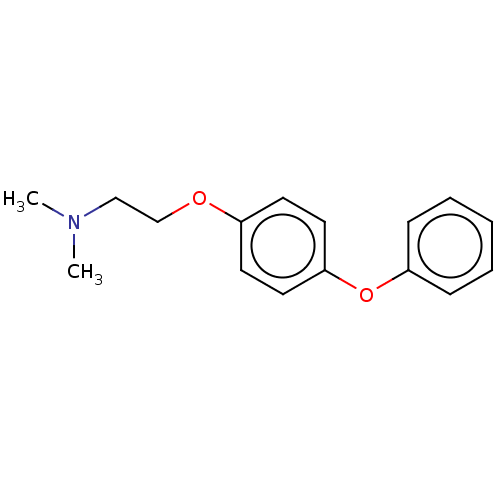
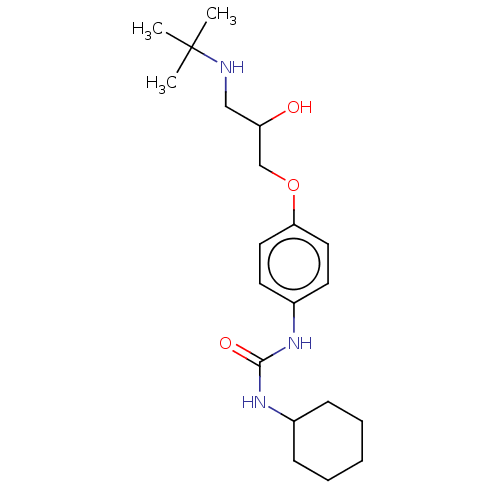
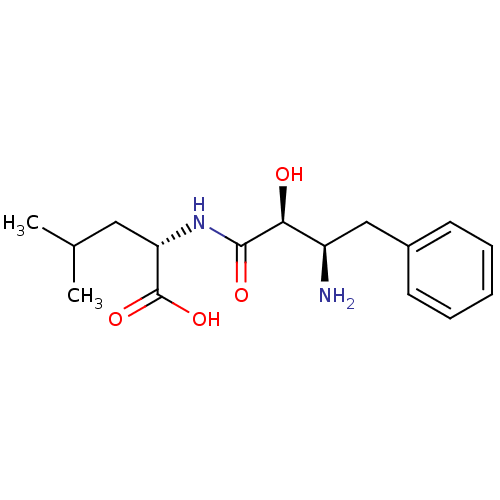
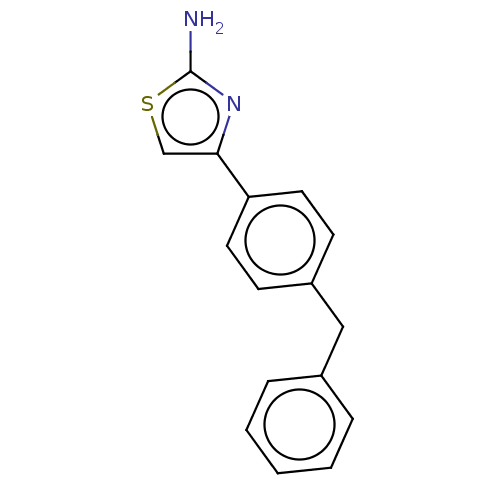
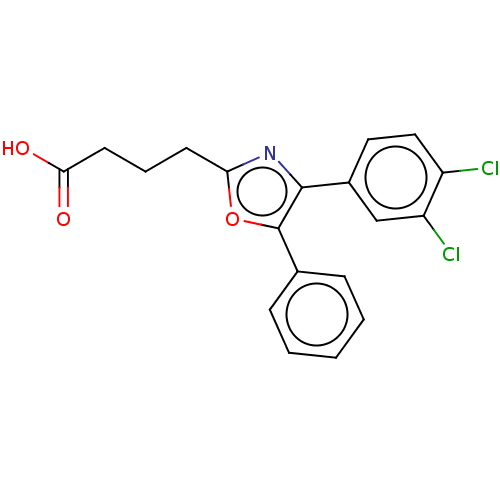
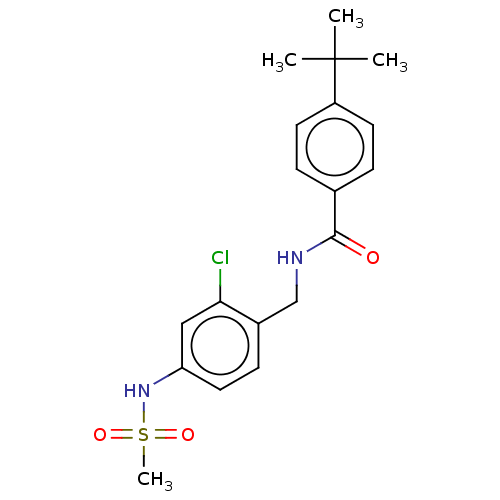
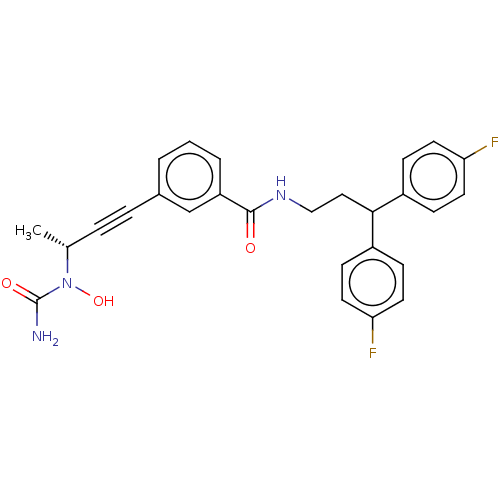
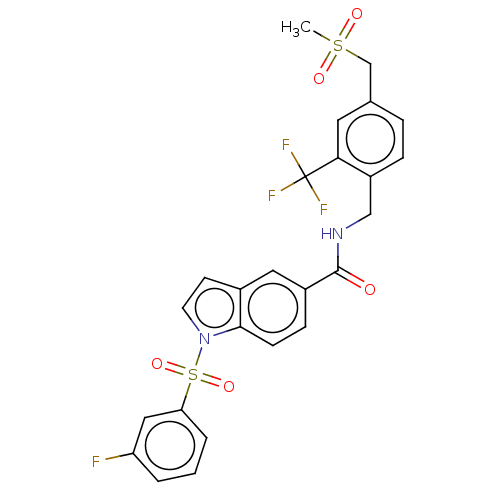
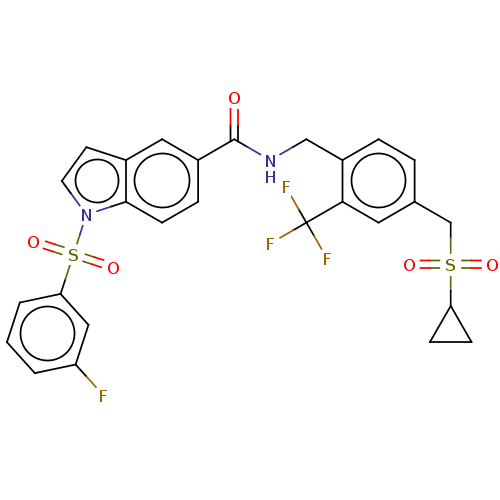
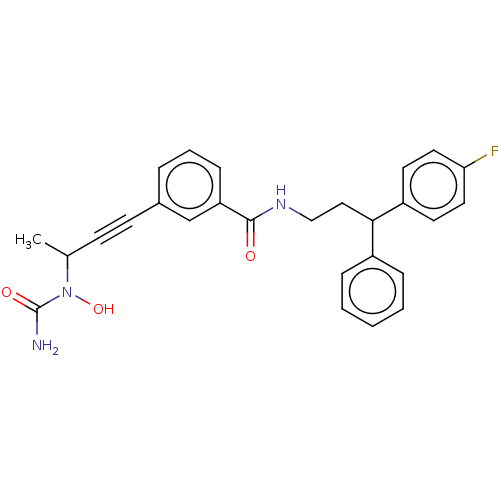
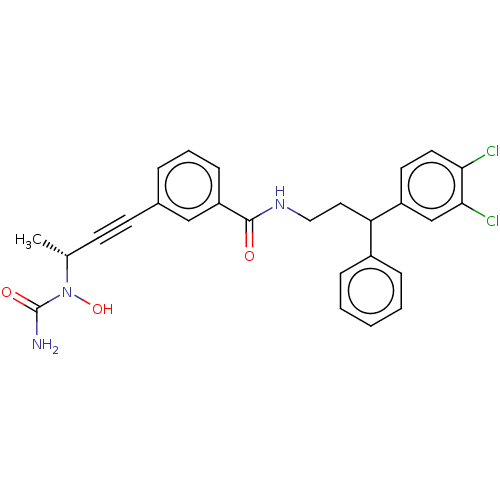
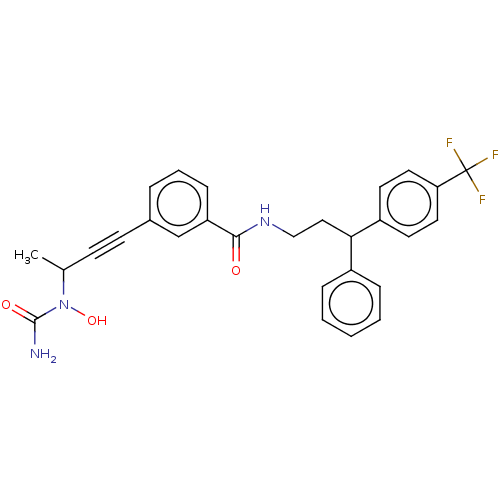
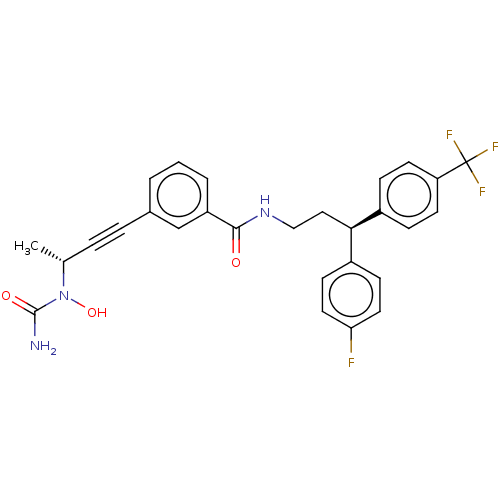
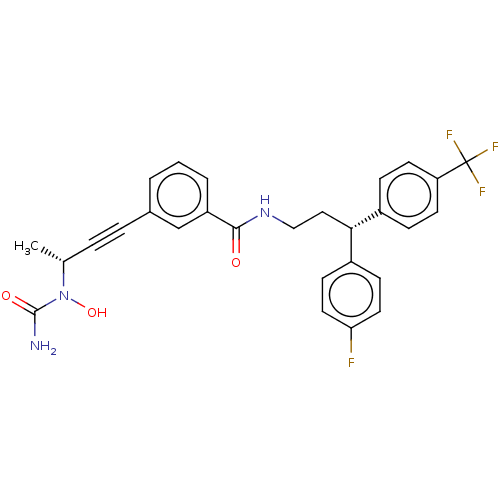
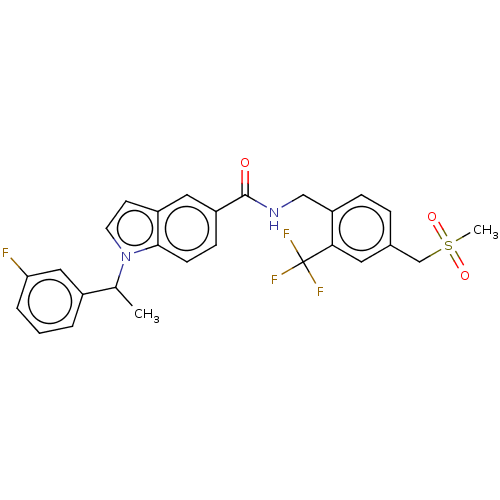
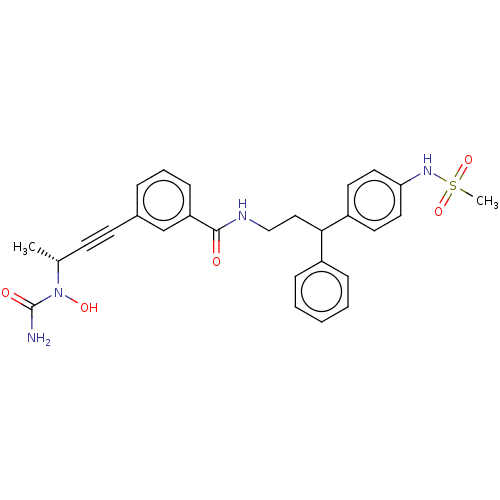
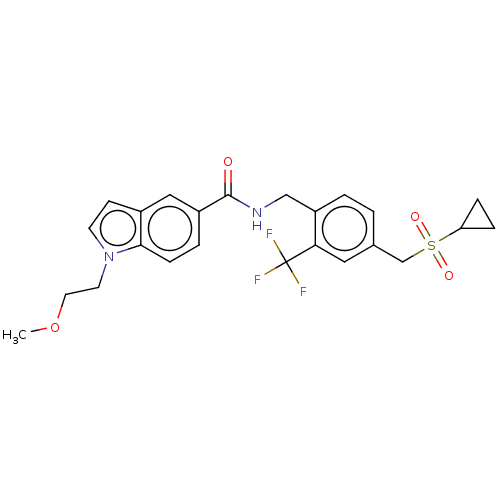
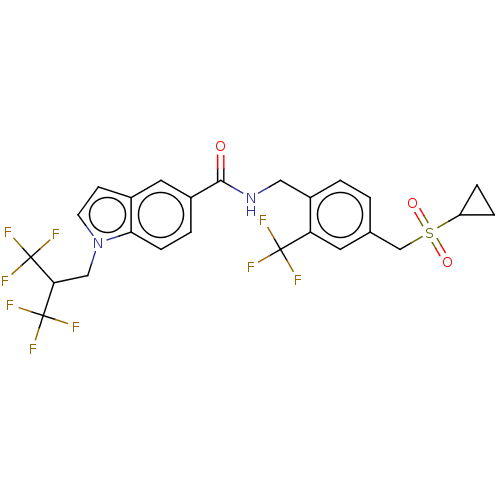
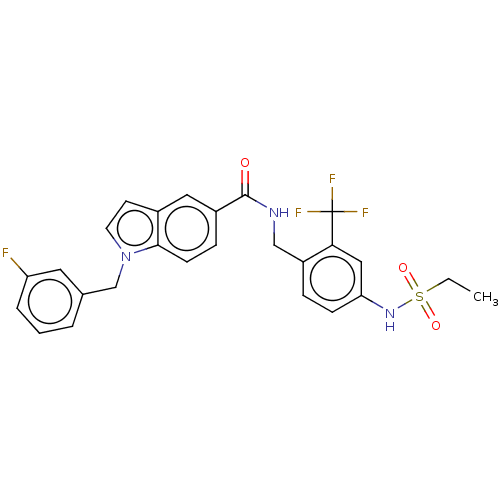
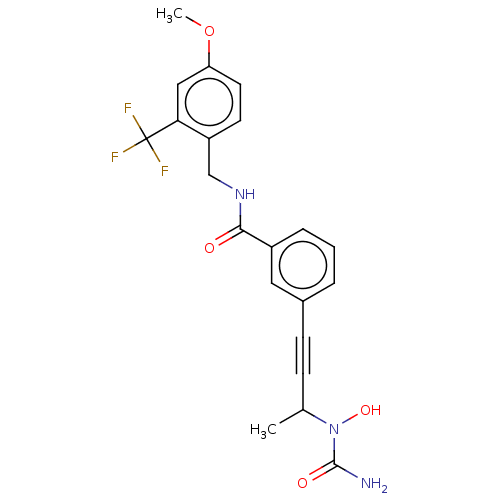
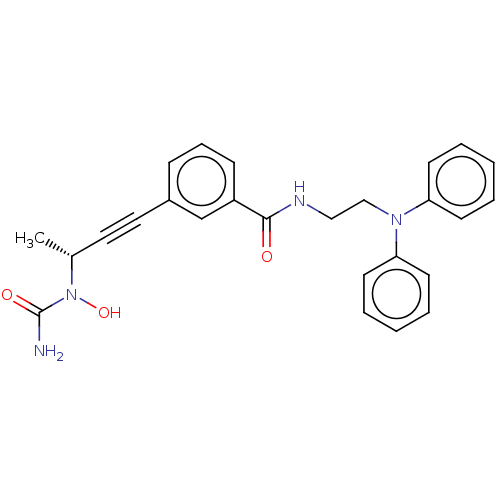
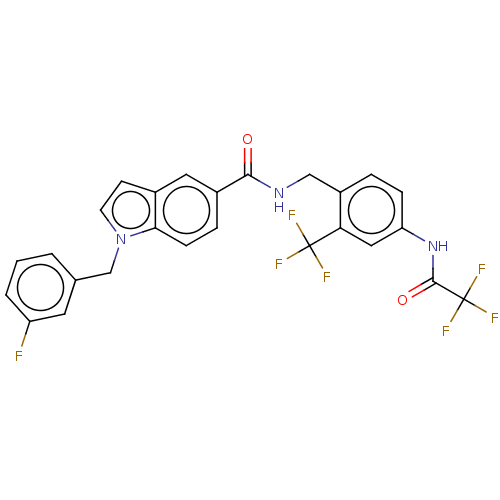
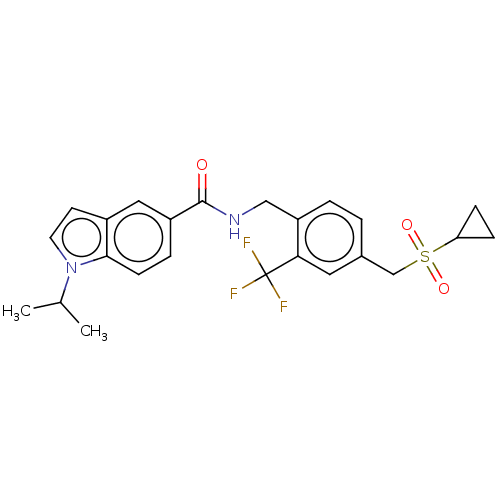
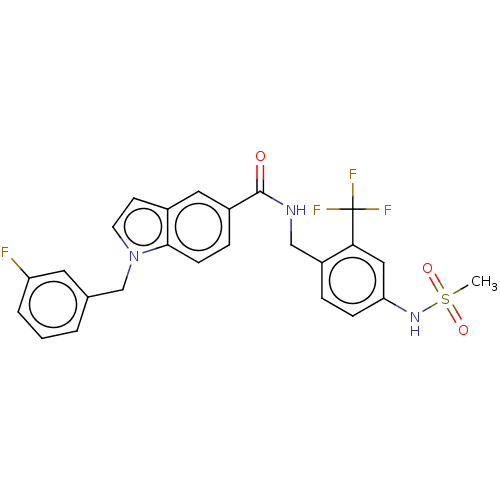
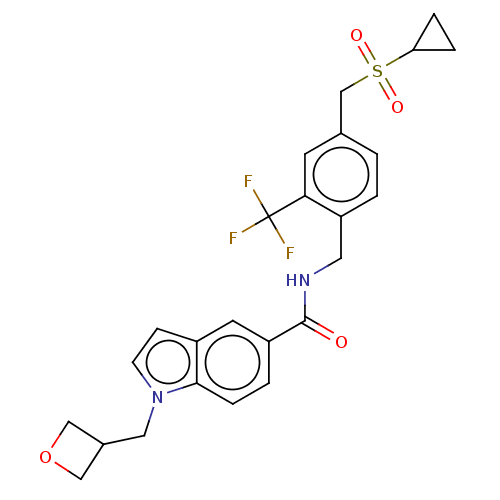
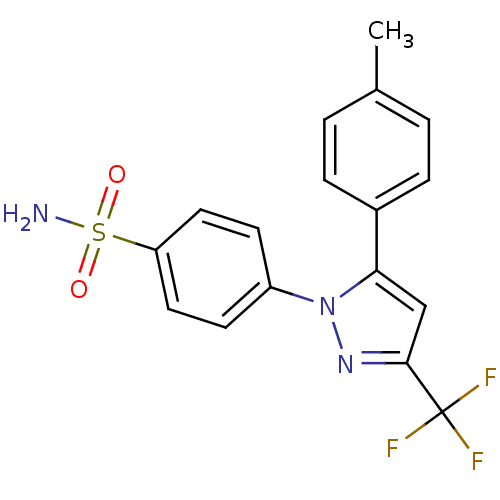
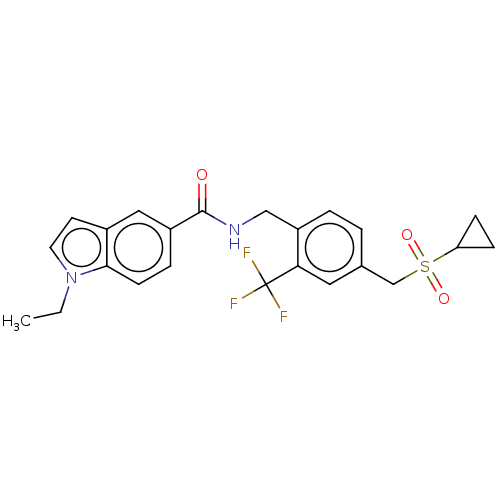
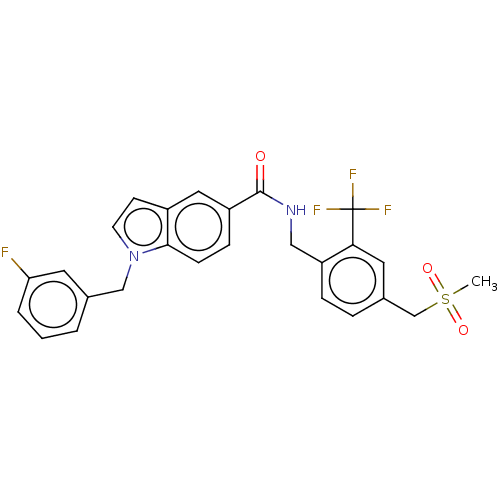
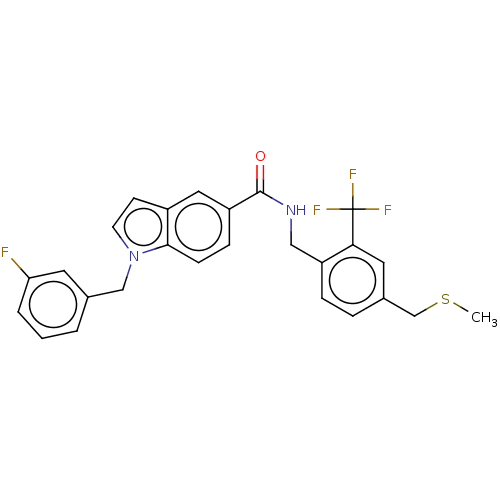
Affinity DataKi: 2nMAssay Description:Displacement of [3H]estradiol from human ERalpha ligand binding domain expressed in Escherichia coli BL21 (DE3)More data for this Ligand-Target Pair
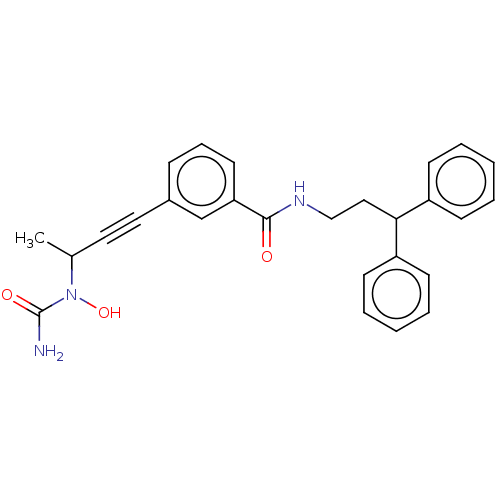
Affinity DataKi: 3nMAssay Description:Non-competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino...More data for this Ligand-Target Pair
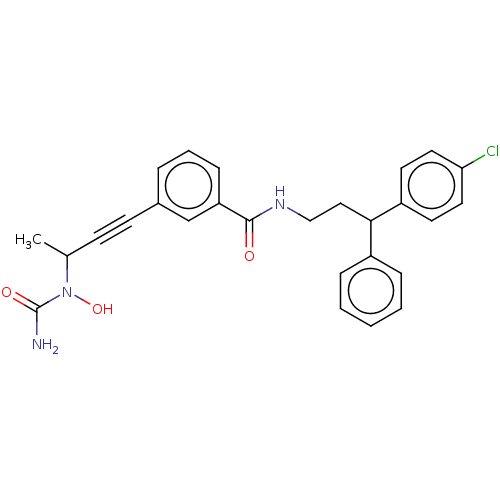
Affinity DataKi: 160nMAssay Description:Competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino-4-M...More data for this Ligand-Target Pair
TargetBeta-1 adrenergic receptor(Homo sapiens (Human))
Goethe-University Of Frankfurt
Curated by ChEMBL
Goethe-University Of Frankfurt
Curated by ChEMBL
Affinity DataKi: 240nMAssay Description:Binding affinity to beta1 adrenergic receptor (unknown origin) by radioligand binding assayMore data for this Ligand-Target Pair
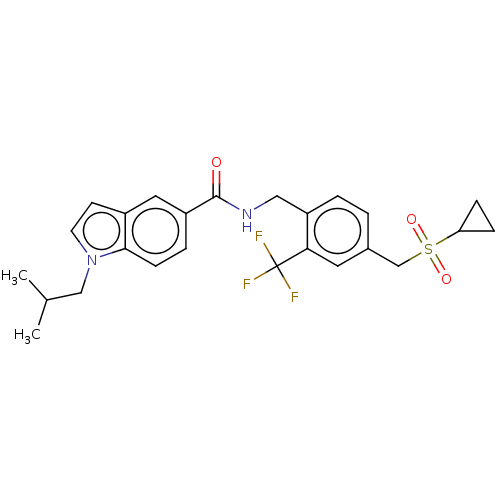
Affinity DataKi: 320nMAssay Description:Competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino-4-M...More data for this Ligand-Target Pair
Affinity DataKi: 360nMAssay Description:Competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino-4-M...More data for this Ligand-Target Pair
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
Goethe-University Of Frankfurt
Curated by ChEMBL
Goethe-University Of Frankfurt
Curated by ChEMBL
Affinity DataKi: 900nMAssay Description:Binding affinity to beta2 adrenergic receptor (unknown origin) by radioligand binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.70E+3nMAssay Description:Competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino-4-M...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataKi: 4.80E+3nMAssay Description:Competitive inhibition of full length human soluble epoxide hydrolase pre-incubated for 30 mins before DiFMUP substrate addition by fluorescence base...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataKi: 4.80E+3nMAssay Description:Competitive inhibition of full length human soluble epoxide hydrolase pre-incubated for 30 mins before DiFMUP substrate addition by fluorescence base...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of sEH in human HepG2 cells using 14(15)-EET-d11 as substrate assessed as reduction in conversion of 14(15)-EET-d11 to 14(15)-DHET-d11 pre...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 2.40nMAssay Description:Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 2.40nMAssay Description:Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 2.70nMAssay Description:Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 4.10nMAssay Description:Inhibition of recombinant v-Abl tyrosine kinase.More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
Johann Wolfgang Goethe-UniversitäT
Curated by ChEMBL
Johann Wolfgang Goethe-UniversitäT
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibitory concentration against COX-2 upon incubation for 15 minutes at 37 degree CMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
Johann Wolfgang Goethe-UniversitäT
Curated by ChEMBL
Johann Wolfgang Goethe-UniversitäT
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibitory concentration against COX-2 upon incubation for 15 minutes at 37 degree CMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair
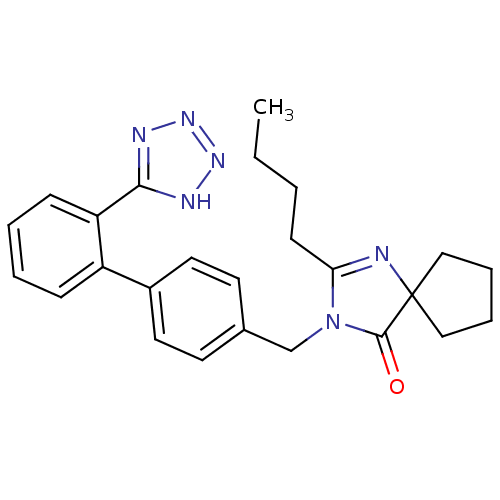
TargetType-1 angiotensin II receptor(Homo sapiens (Human))
Fraunhofer Institute For Translational Medicine And Phamacology Itmp
Curated by ChEMBL
Fraunhofer Institute For Translational Medicine And Phamacology Itmp
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Antagonist activity at human AT1 overexpressed in CHO-K1 cells in presence of 10 nM [Val5-angiotensin II measured after 90 mins by HTRF based IP-one ...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using L-arginine-7-amino-4-Methylcoumarine as substrate pr...More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)