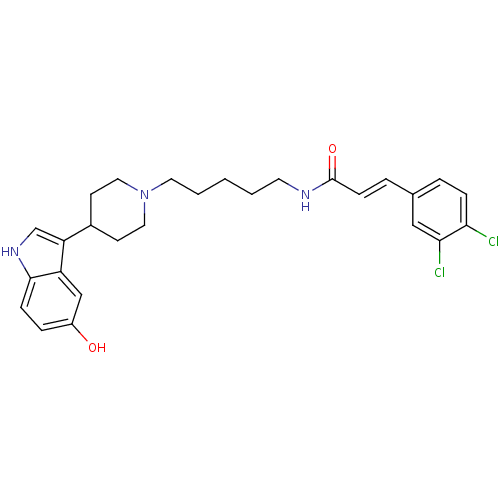
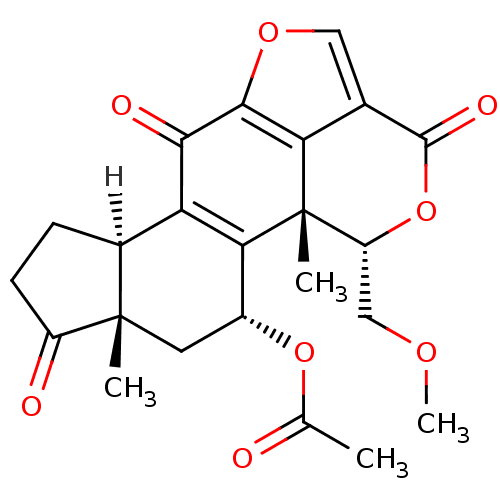
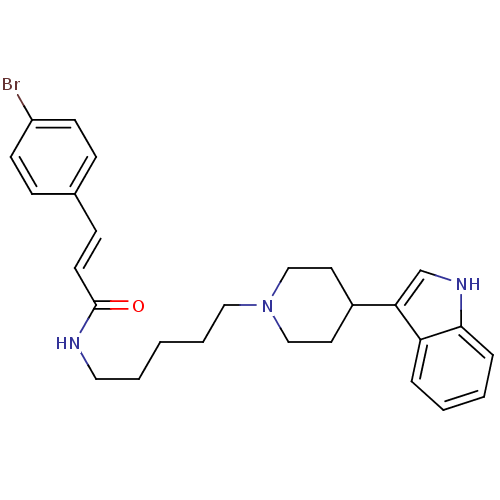
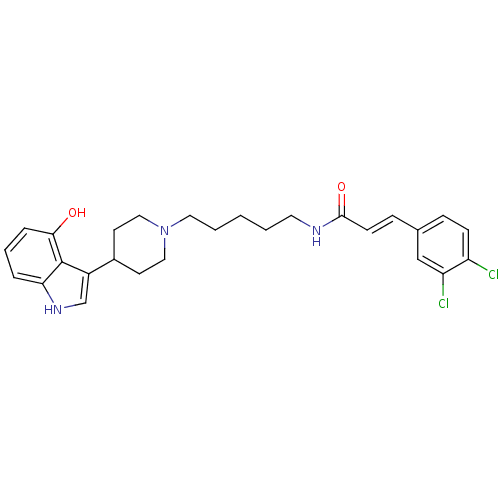
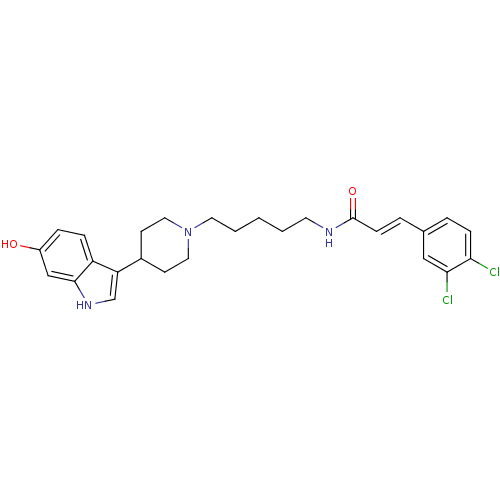
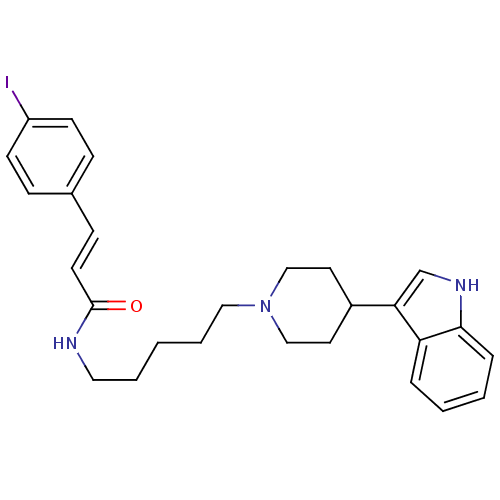
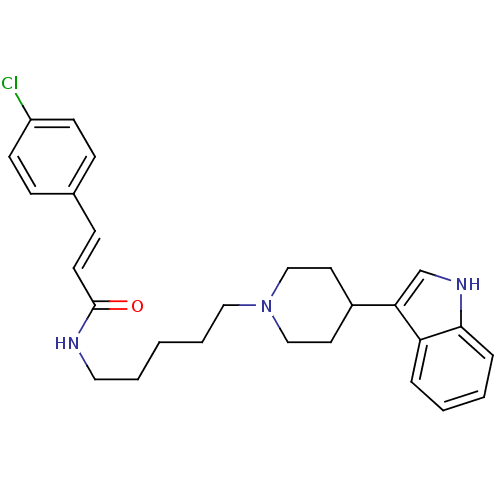
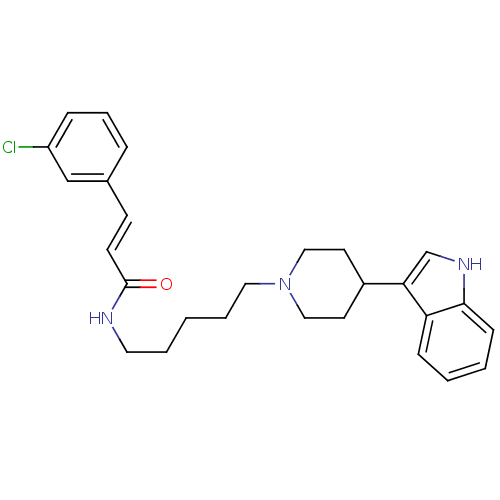
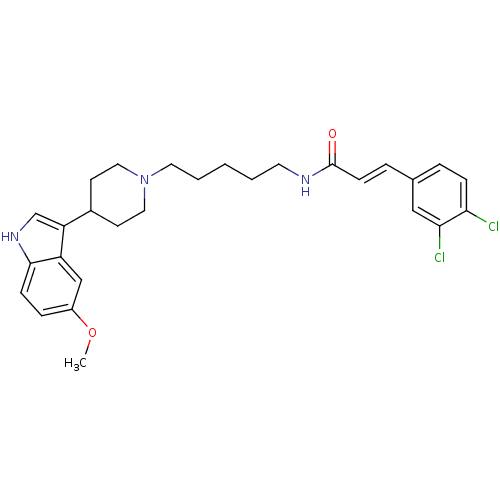
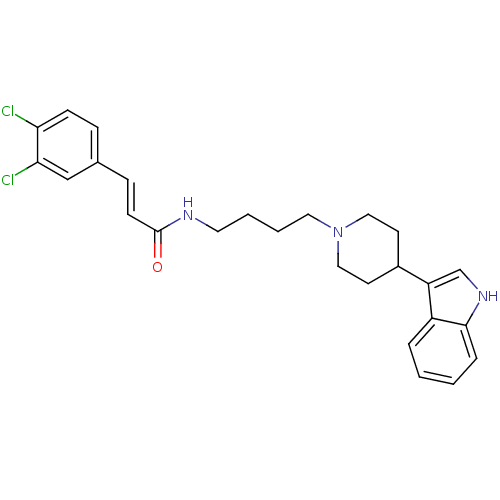
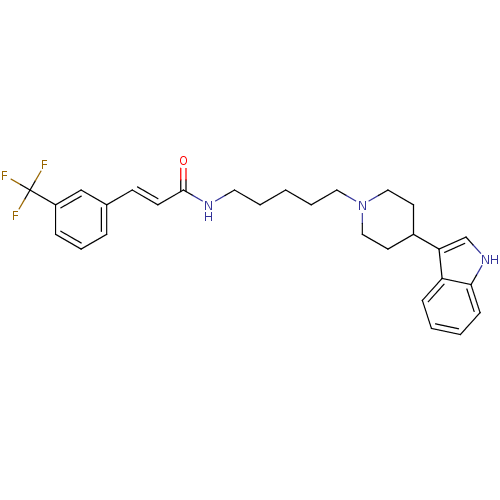
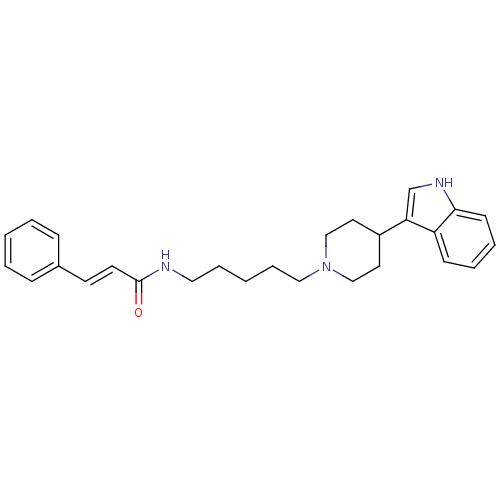
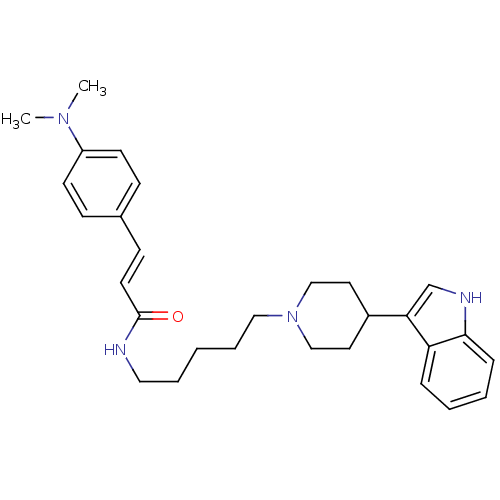
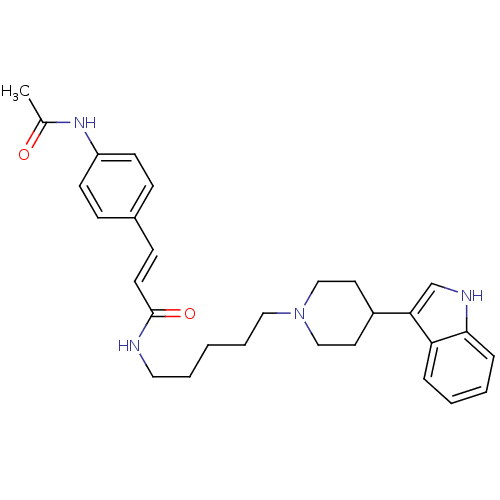
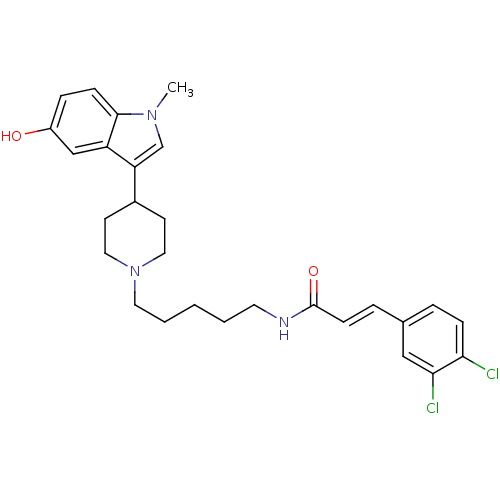
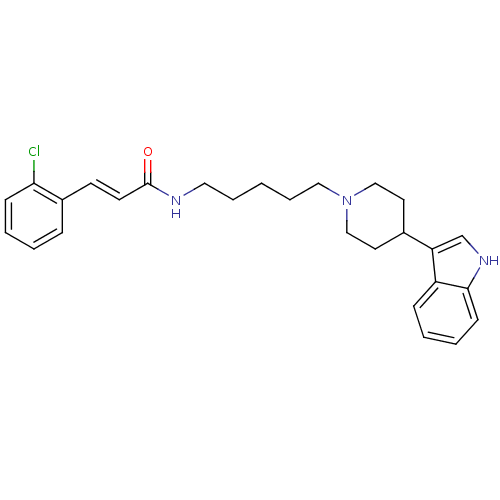
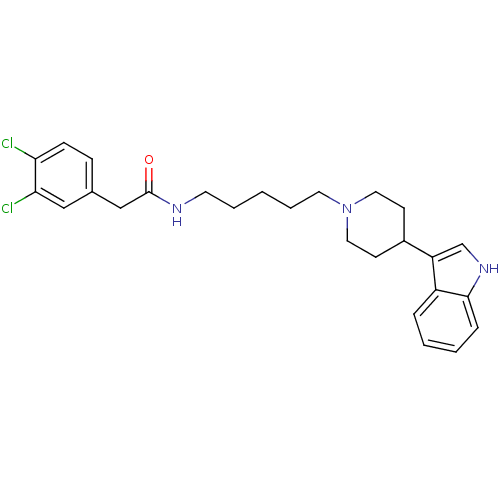
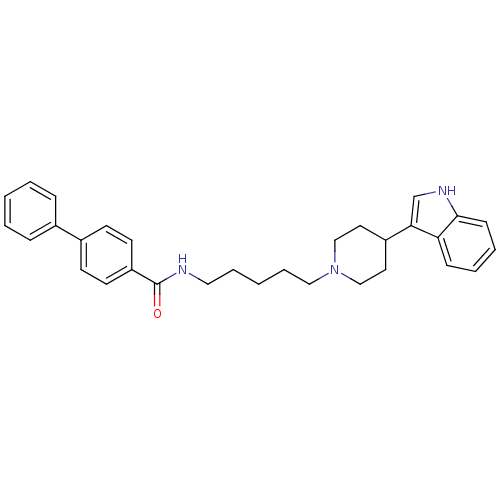
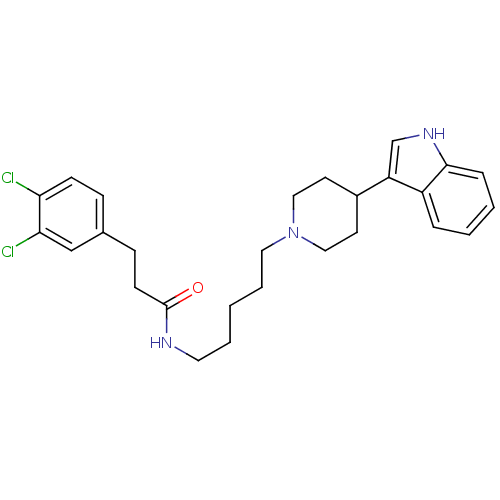
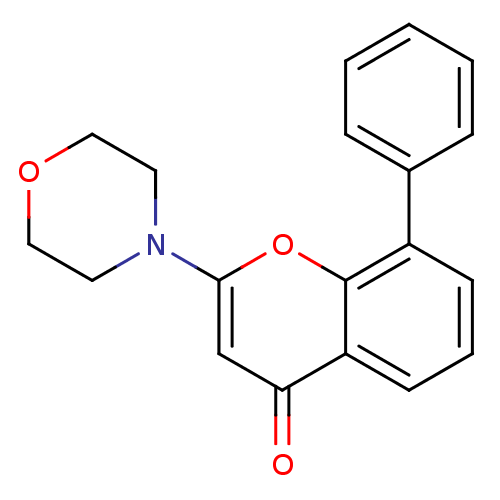
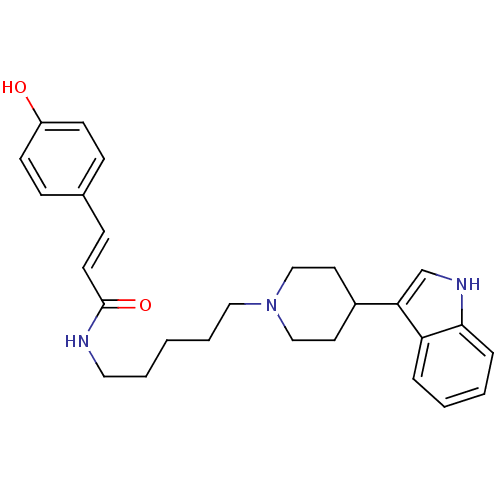
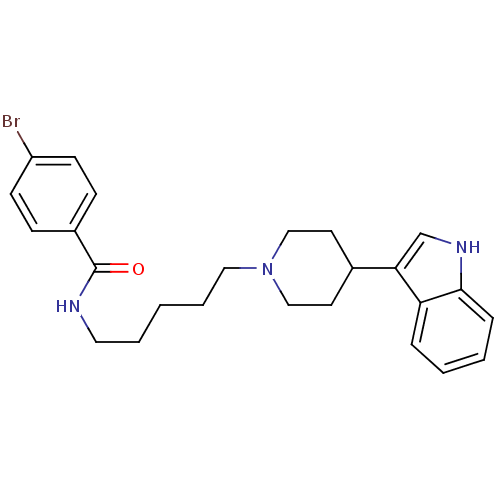
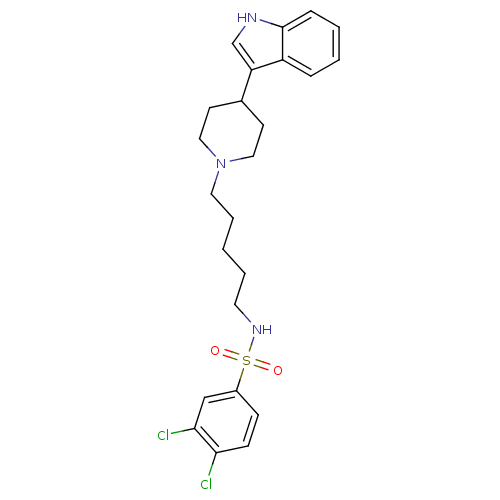
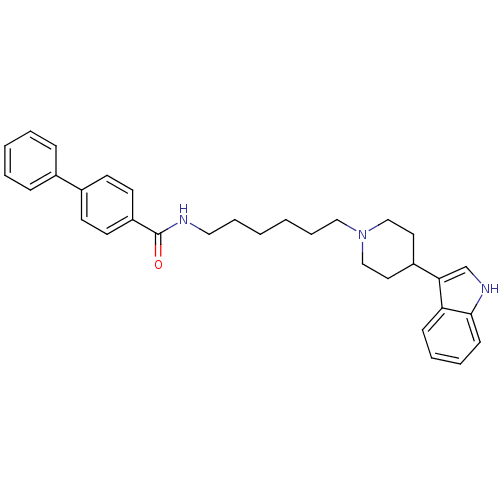
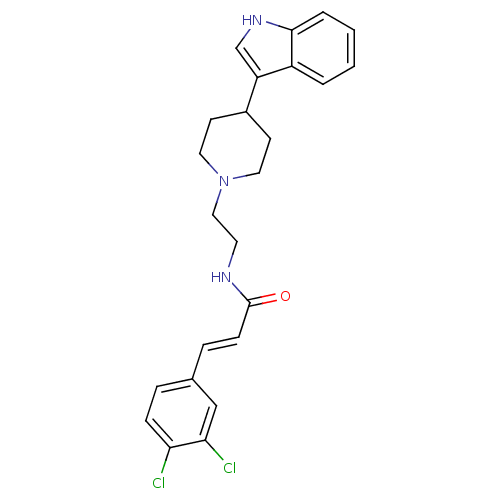
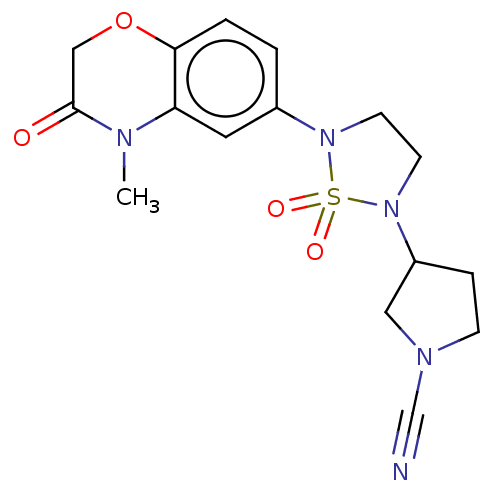
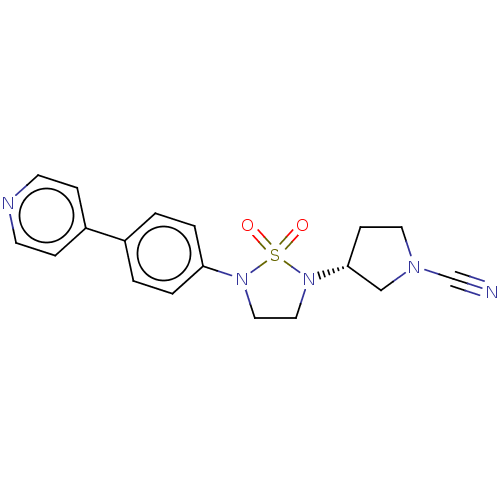
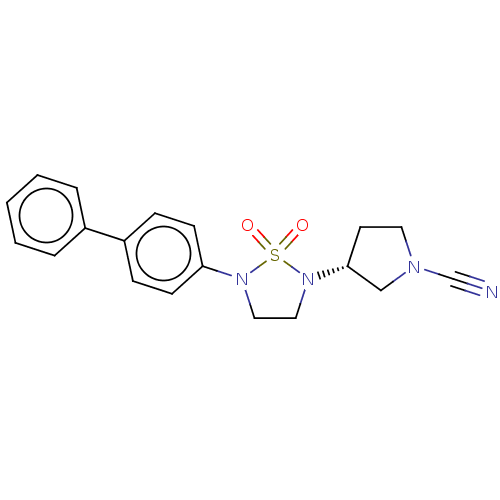
TargetDNA-dependent protein kinase catalytic subunit(Homo sapiens (Human))
School Of Natural Sciences--Chemistry
Curated by ChEMBL
School Of Natural Sciences--Chemistry
Curated by ChEMBL
Affinity DataKi: 0.650nMAssay Description:Inhibitory activity against DNA-dependent protein kinase receptorMore data for this Ligand-Target Pair
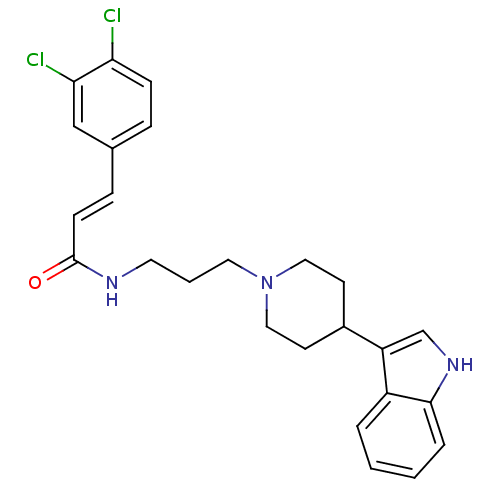
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 50nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
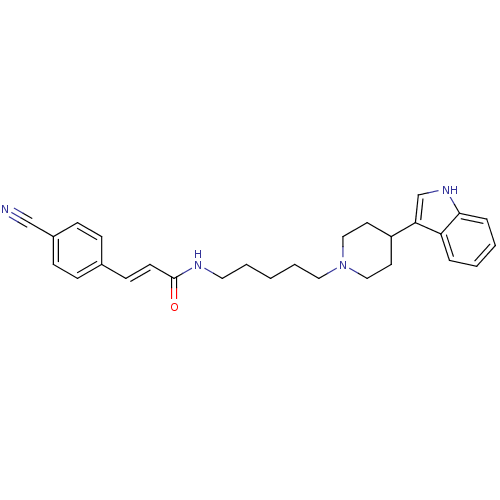
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 79nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
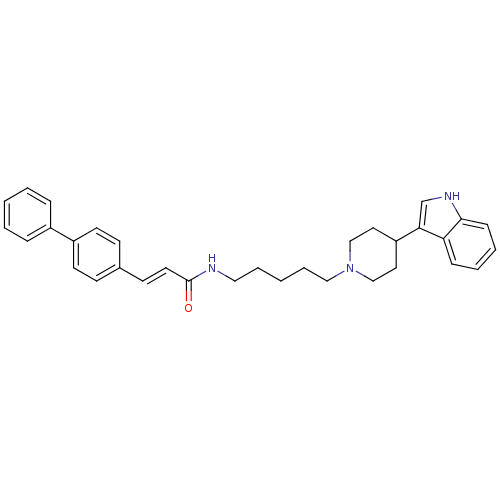
TargetDNA-dependent protein kinase catalytic subunit(Homo sapiens (Human))
School Of Natural Sciences--Chemistry
Curated by ChEMBL
School Of Natural Sciences--Chemistry
Curated by ChEMBL
Affinity DataKi: 120nMAssay Description:Binding affinity for DNA dependent protein kinase isolated from HeLa cells; Range is 20-120More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 120nMAssay Description:Inhibition of human recombinant p110 alpha Phosphatidylinositol 3-kinaseMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 234nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 350nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 360nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 360nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 420nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 460nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 520nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 550nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 560nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 590nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 660nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 790nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 790nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 870nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 1.20E+3nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 1.45E+3nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 1.60E+3nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 1.70E+3nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 2.50E+3nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 2.80E+3nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 2.90E+3nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 4.20E+3nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 4.26E+3nMAssay Description:Compound was evaluated for the antagonist activity against C-C chemokine receptor type 5More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 4.40E+3nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 4.40E+3nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 5.10E+3nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 5.30E+3nMAssay Description:Compound was evaluated for the antagonist activity against human C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 5.40E+3nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 5.90E+3nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 6.00E+3nMAssay Description:Binding affinity for Phosphatidylinositol-3-kinase isolated from HeLa cells; Range is 20-120More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 6.30E+3nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 7.10E+3nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Antagonist activity against C-C chemokine receptor type 2More data for this Ligand-Target Pair
Affinity DataIC50: <0.100nMAssay Description:Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po...More data for this Ligand-Target Pair
Affinity DataIC50: <0.100nMAssay Description:Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po...More data for this Ligand-Target Pair
Affinity DataIC50: <0.100nMAssay Description:Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po...More data for this Ligand-Target Pair
Affinity DataIC50: <0.100nMAssay Description:Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po...More data for this Ligand-Target Pair
Affinity DataIC50: <0.100nMAssay Description:Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po...More data for this Ligand-Target Pair
Affinity DataIC50: <0.100nMAssay Description:Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po...More data for this Ligand-Target Pair
Affinity DataIC50: <0.100nMAssay Description:Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po...More data for this Ligand-Target Pair
Affinity DataIC50: <0.100nMAssay Description:Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po...More data for this Ligand-Target Pair
Affinity DataIC50: <0.100nMAssay Description:Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po...More data for this Ligand-Target Pair
Affinity DataIC50: <0.100nMAssay Description:Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po...More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)