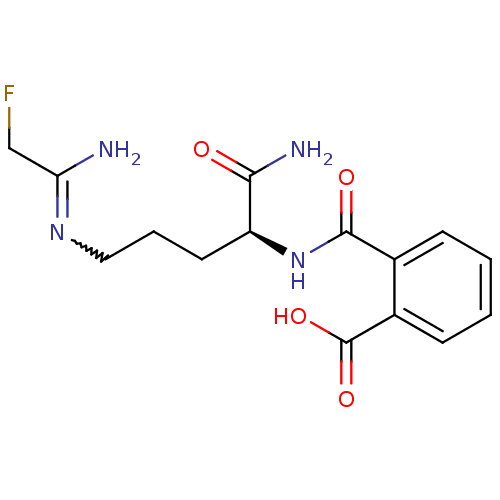
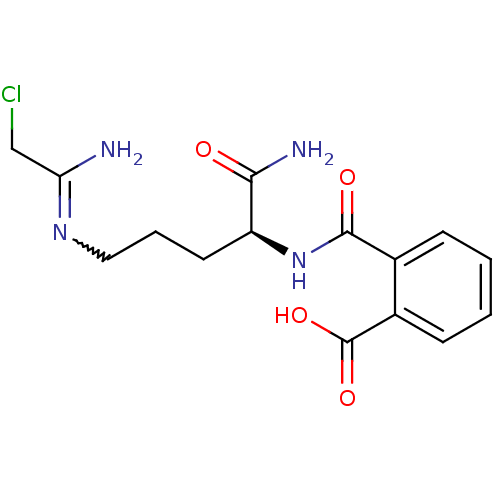
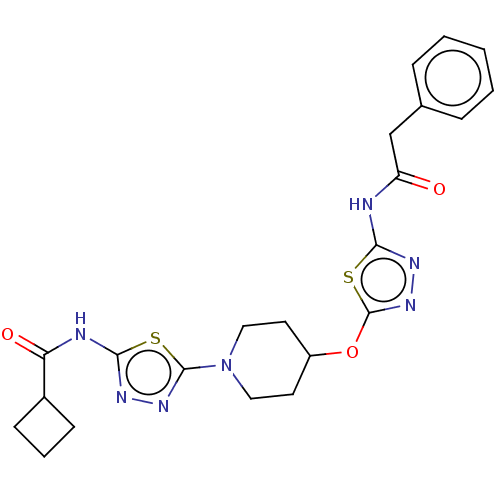
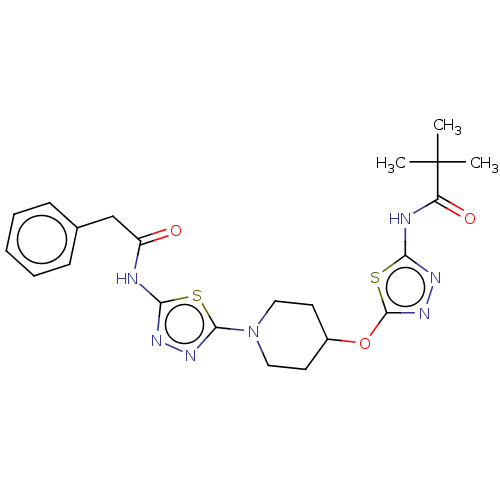
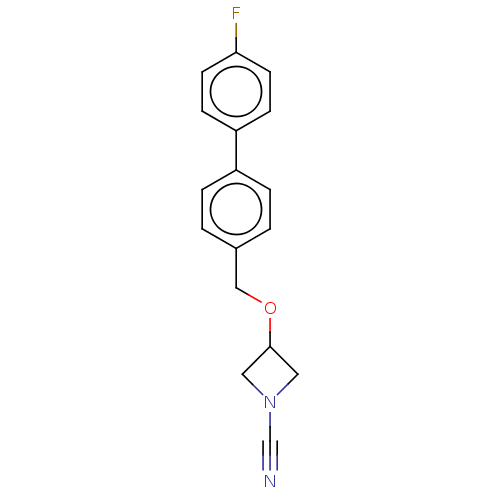
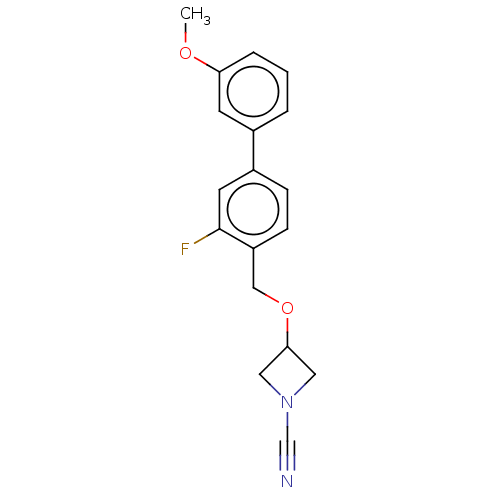
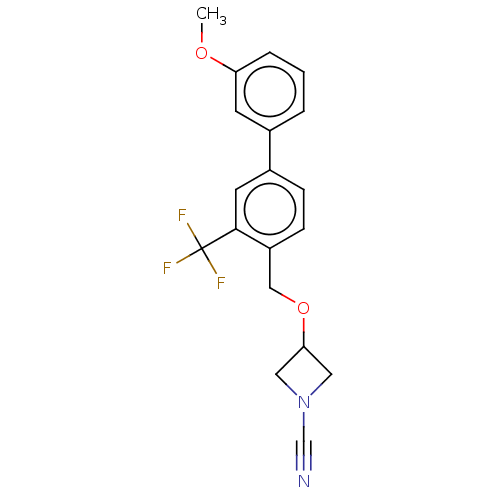
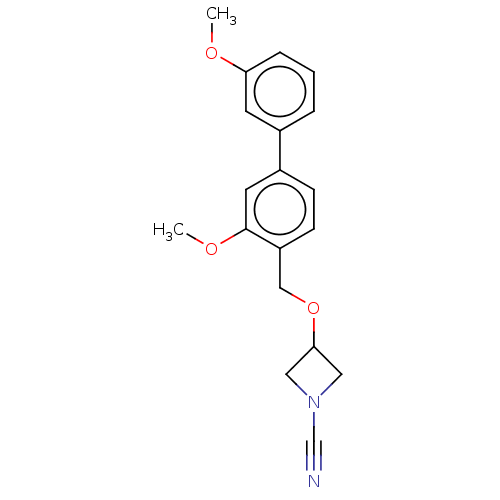
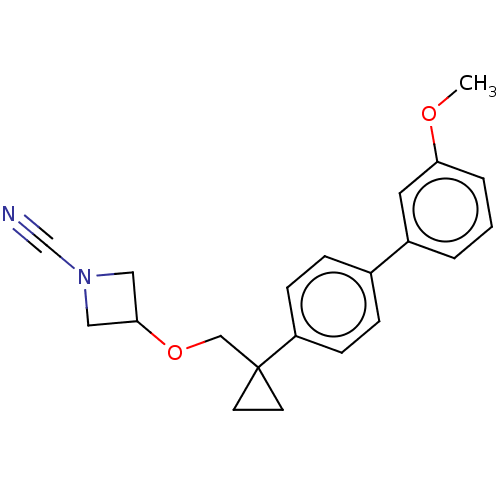
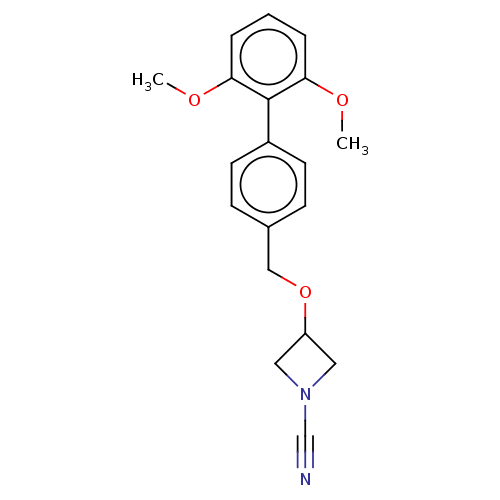
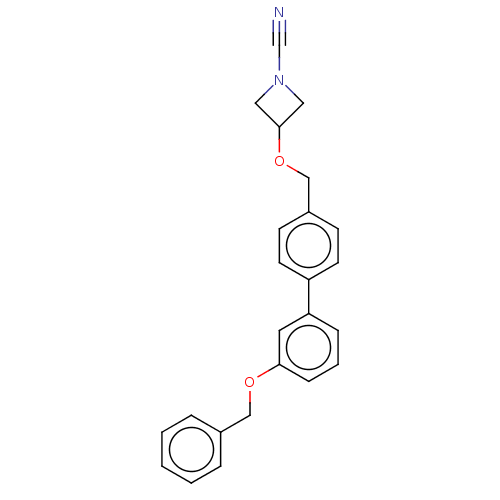
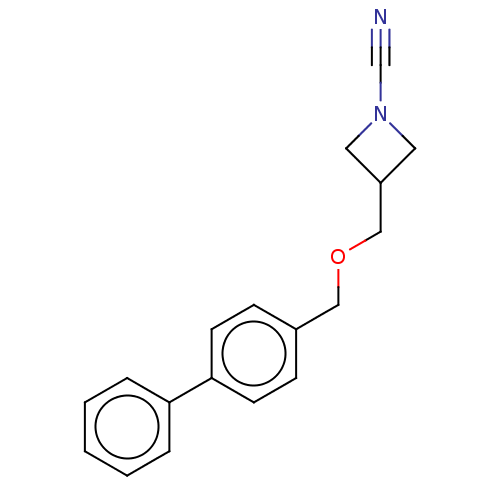
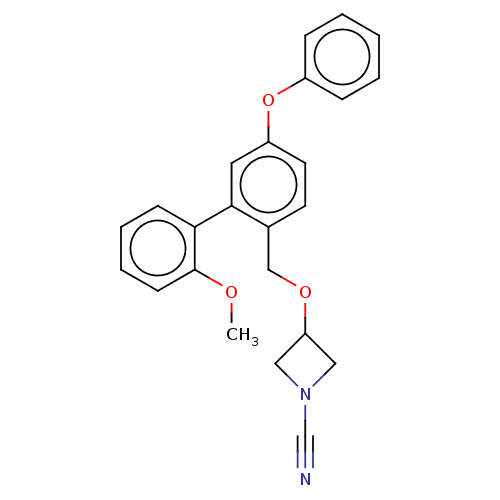
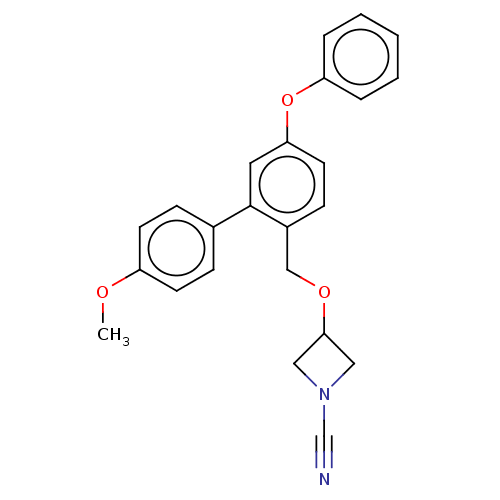
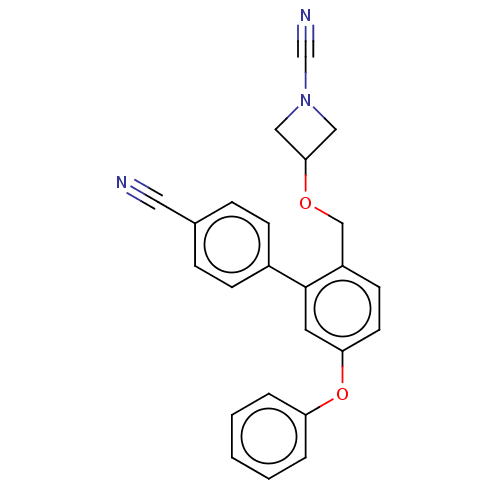
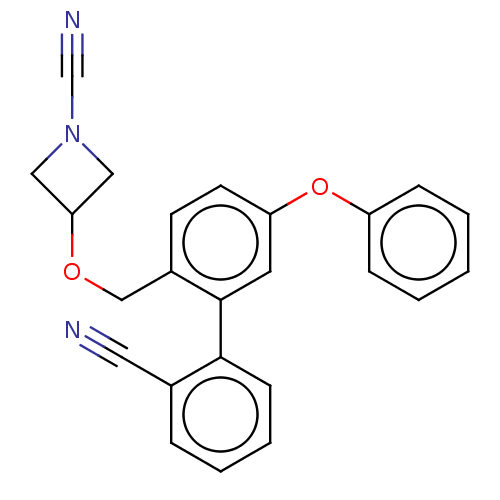
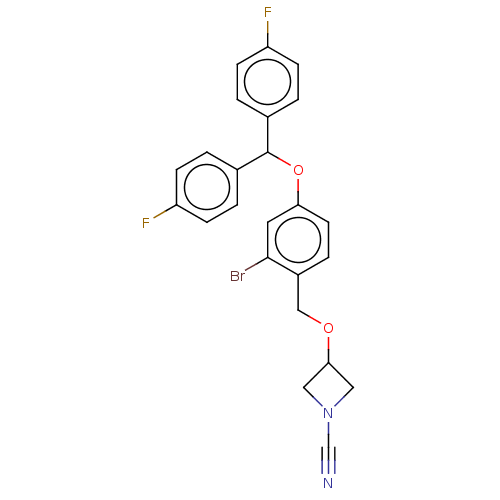
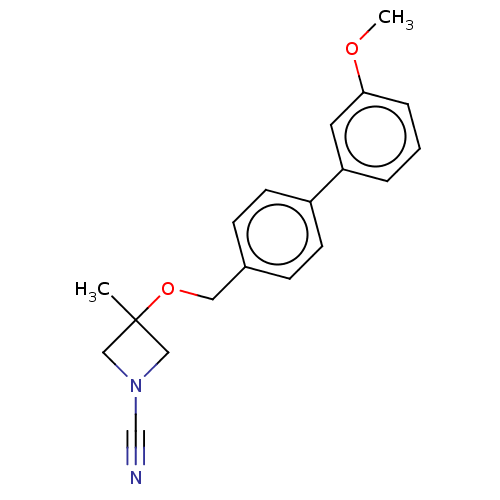
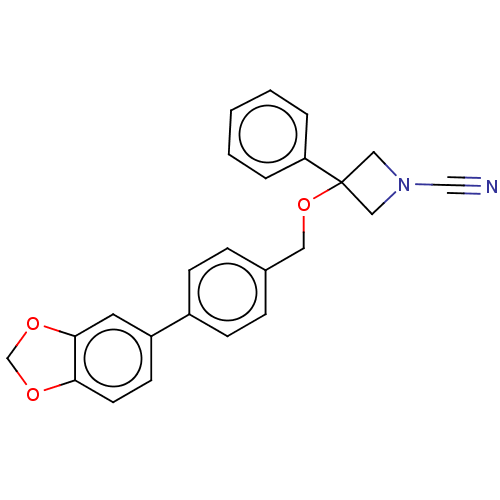
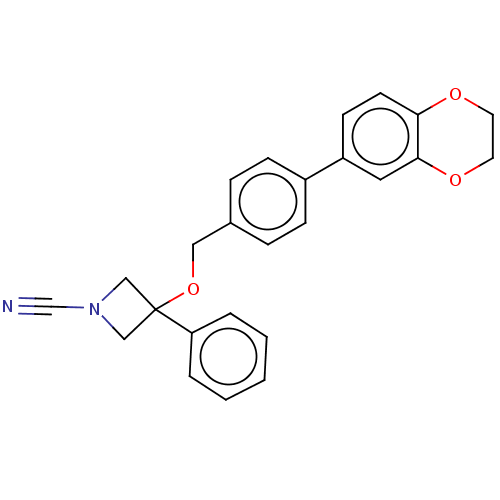
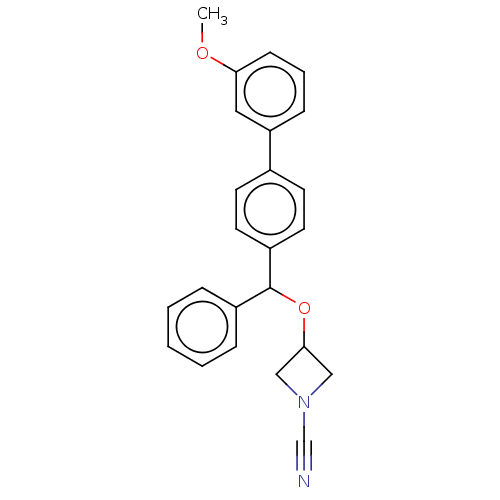
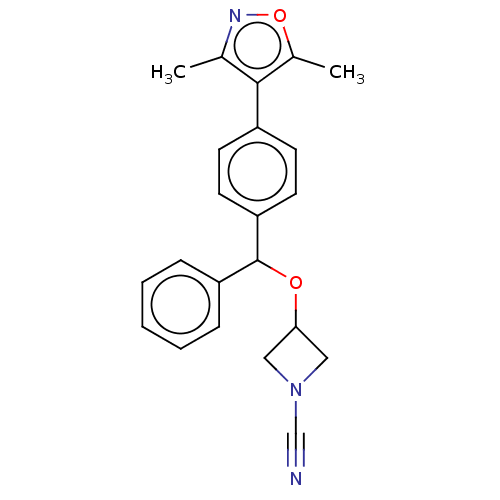
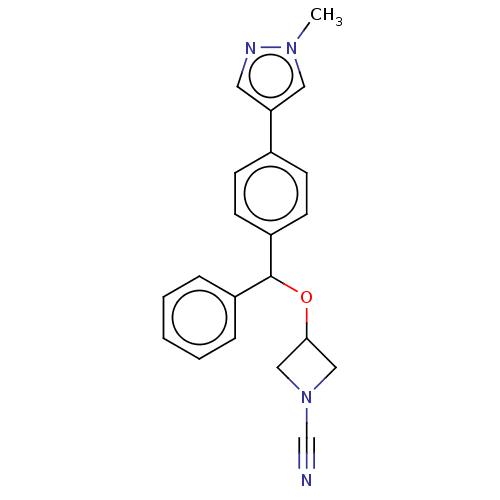
TargetProtein-arginine deiminase type-2(Homo sapiens (Human))
The Scripps Research Institute-Florida
Curated by ChEMBL
The Scripps Research Institute-Florida
Curated by ChEMBL
Affinity DataKi: 8.08E+3nMAssay Description:Inhibition of recombinant wild-type PAD2 (unknown origin) using N-alpha-Benzoyl-L-arginine ethyl ester as substrate preincubated for 10 mins followed...More data for this Ligand-Target Pair
TargetProtein-arginine deiminase type-1(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 9.40E+3nMAssay Description:Irreversible inhibition of PAD1 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet...More data for this Ligand-Target Pair
TargetProtein-arginine deiminase type-4(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 1.30E+4nMAssay Description:Irreversible inhibition of PAD4 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet...More data for this Ligand-Target Pair
TargetProtein-arginine deiminase type-4(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 1.60E+4nMAssay Description:Irreversible inhibition of PAD4 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet...More data for this Ligand-Target Pair
TargetProtein-arginine deiminase type-4(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 2.01E+4nMAssay Description:Inhibition of recombinant wild-type PAD4 (unknown origin) using N-alpha-Benzoyl-L-arginine ethyl ester as substrate preincubated for 10 mins followed...More data for this Ligand-Target Pair
TargetProtein-arginine deiminase type-3(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 2.80E+4nMAssay Description:Irreversible inhibition of PAD3 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet...More data for this Ligand-Target Pair
TargetProtein-arginine deiminase type-3(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 2.90E+4nMAssay Description:Irreversible inhibition of PAD3 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet...More data for this Ligand-Target Pair
TargetProtein-arginine deiminase type-1(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 6.20E+4nMAssay Description:Irreversible inhibition of PAD1 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet...More data for this Ligand-Target Pair
TargetProtein-arginine deiminase type-1(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 1.10E+5nMAssay Description:Irreversible inhibition of PAD1 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet...More data for this Ligand-Target Pair
TargetProtein-arginine deiminase type-4(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 1.80E+5nMAssay Description:Irreversible inhibition of PAD4 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet...More data for this Ligand-Target Pair
TargetProtein-arginine deiminase type-3(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 2.93E+5nMAssay Description:Irreversible inhibition of PAD3 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet...More data for this Ligand-Target Pair
TargetProtein-arginine deiminase type-4(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 3.30E+5nMAssay Description:Irreversible inhibition of PAD4 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 minsMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 minsMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 minsMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 minsMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataIC50: 27nMAssay Description:Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 minsMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataIC50: 27nMAssay Description:Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 minsMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataIC50: 29nMAssay Description:Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 minsMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataIC50: 29nMAssay Description:Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 minsMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataIC50: 38nMAssay Description:Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 minsMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
University Of Pittsburgh
Curated by ChEMBL
University Of Pittsburgh
Curated by ChEMBL
Affinity DataIC50: 38nMAssay Description:Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 minsMore data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Northeastern University
US Patent
Northeastern University
US Patent
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair