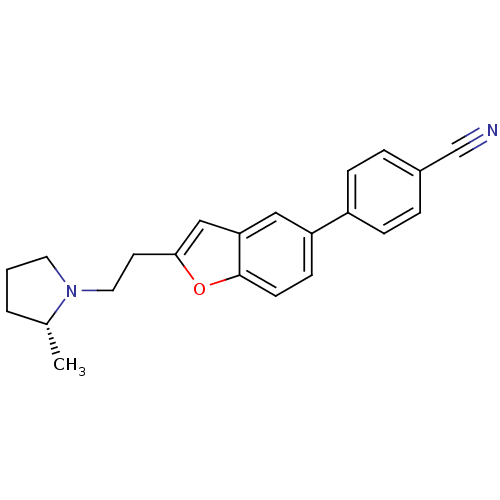
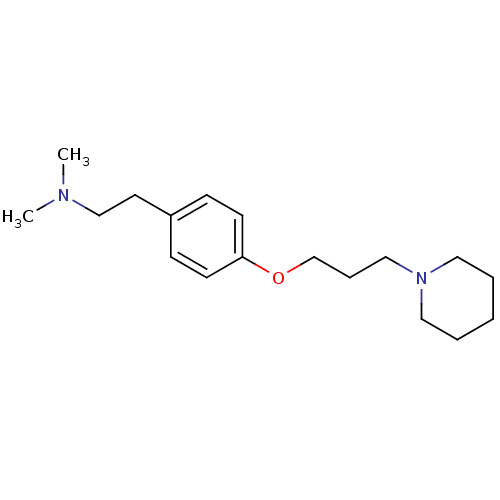
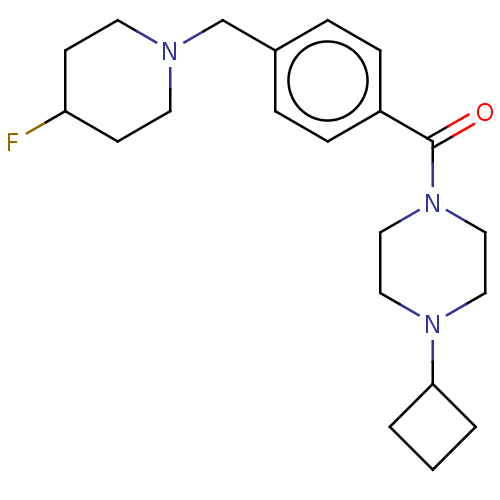
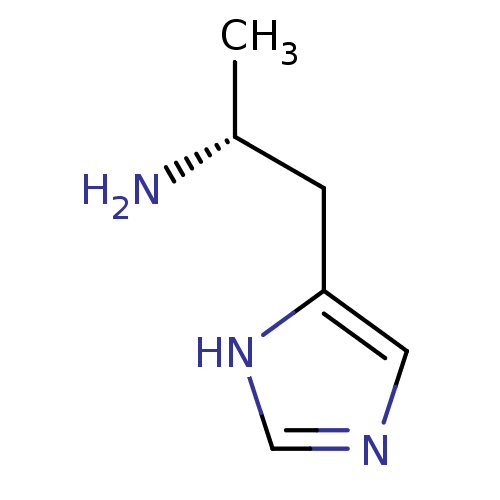
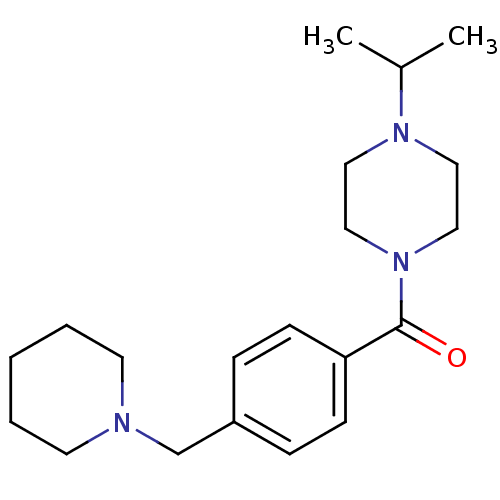
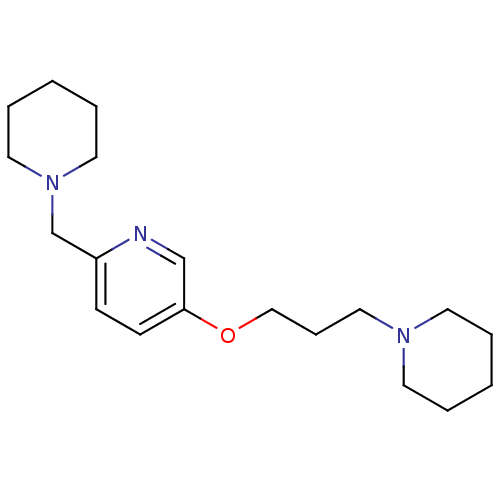
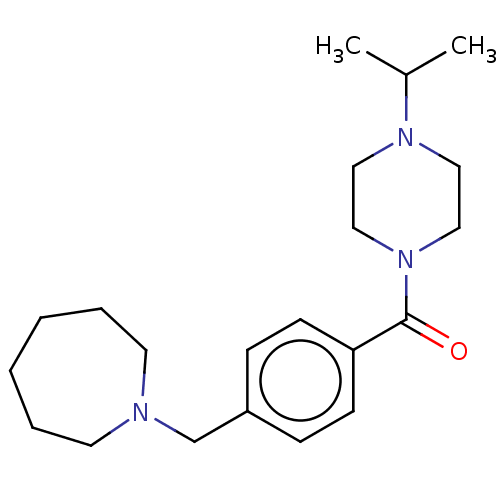
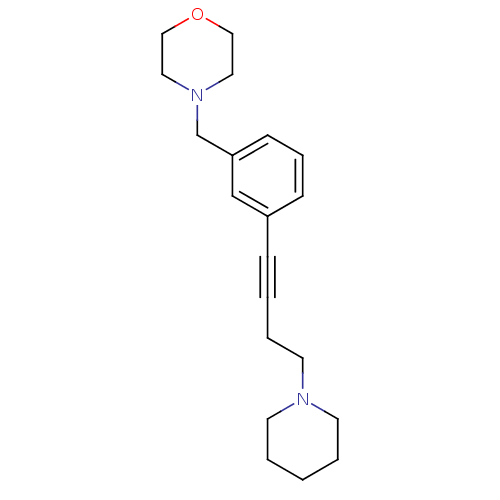
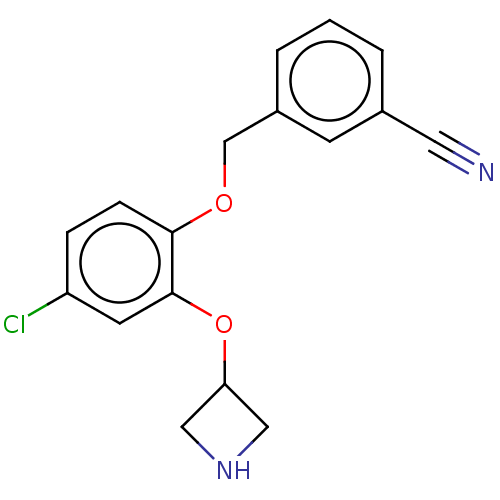
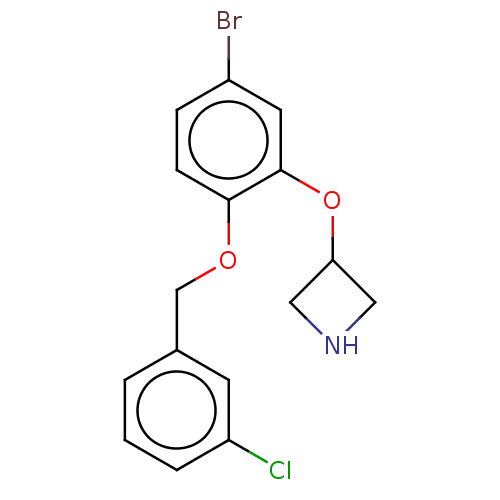
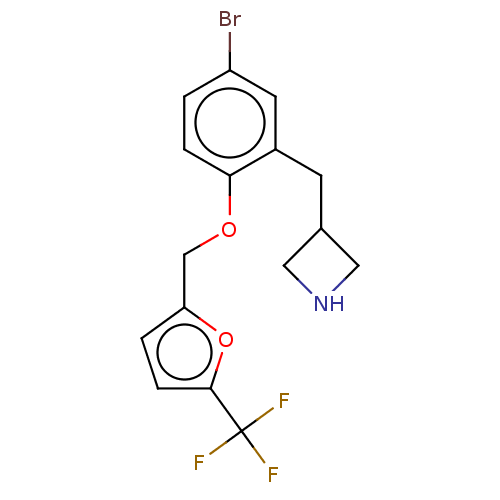
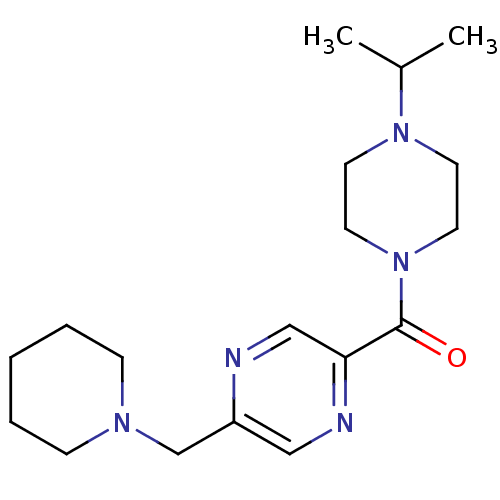
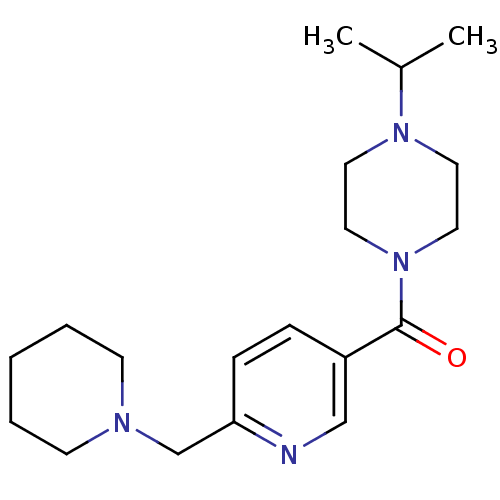
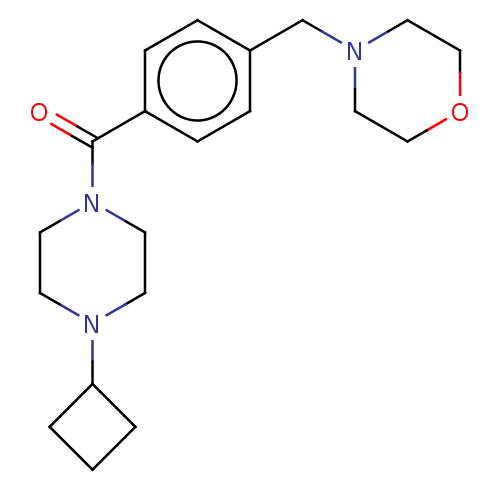
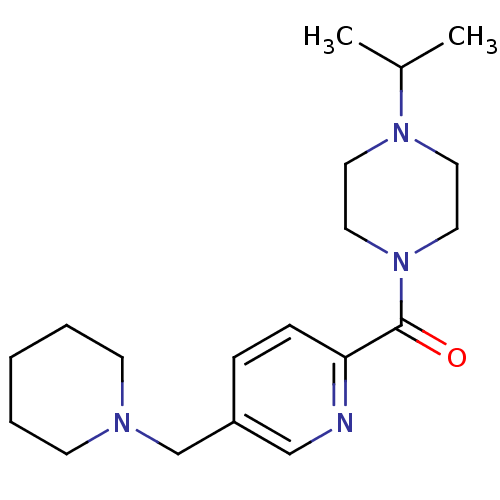
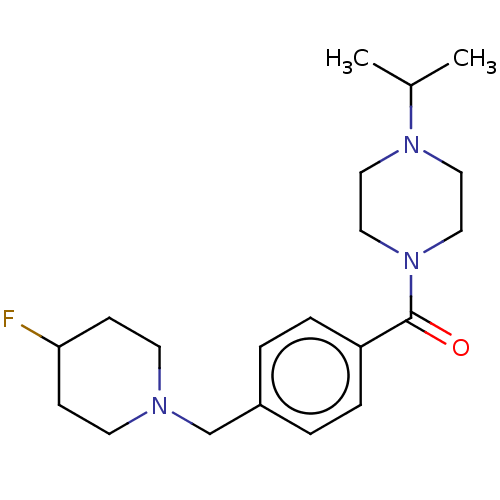
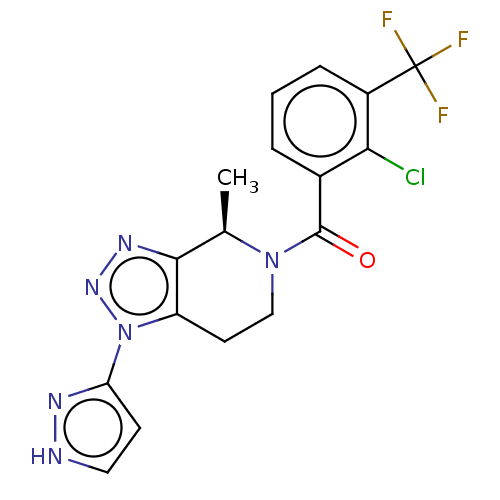
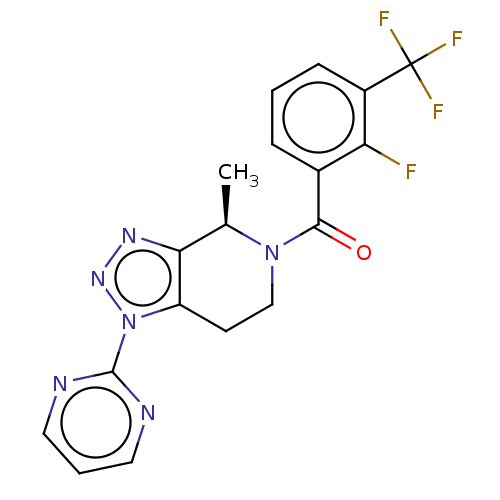
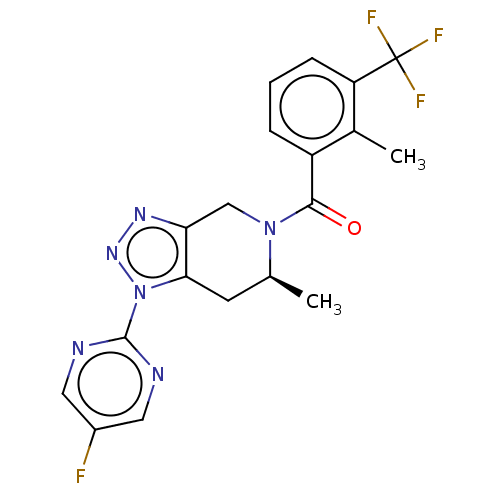
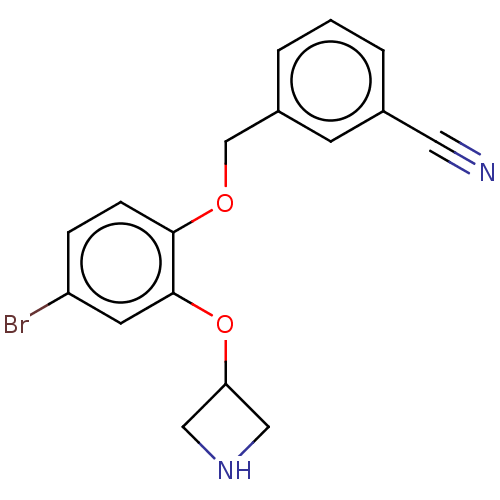
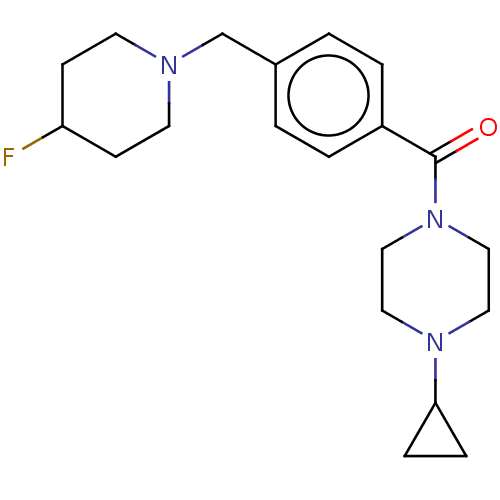
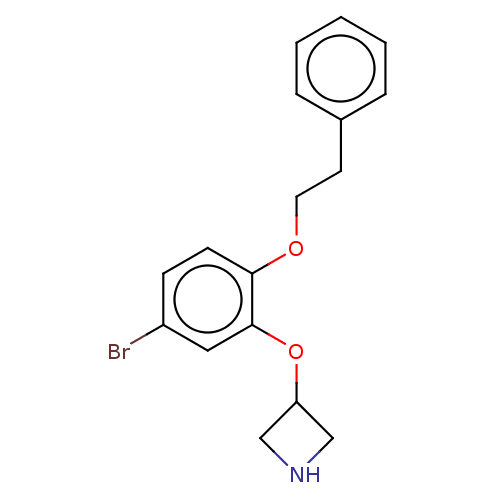
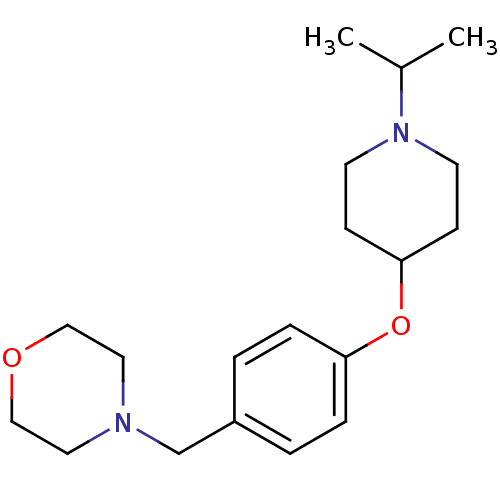
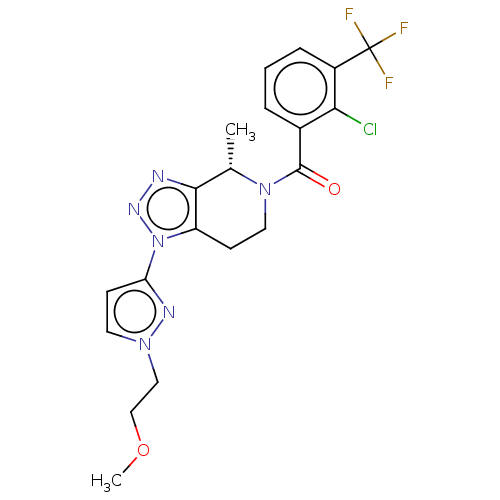
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 0.210nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 0.400nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 0.450nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 0.600nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 minsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 0.900nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 minsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Receptor binding was performed using membrane fractions prepared from the HEK-293 cell line recombinantly expressing rat 5-HT7 receptors (NCBI access...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Receptor binding was performed using membrane fractions prepared from the HEK-293 cell line recombinantly expressing rat 5-HT7 receptors (NCBI access...More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 minsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Displacement of N-[3H]methylhistamine from rat histamine H3 receptor in rat cortical hemispheresMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Receptor binding was performed using membrane fractions prepared from the HEK-293 cell line recombinantly expressing rat 5-HT7 receptors (NCBI access...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t...More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.20nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:human or rat P2X7-1321 N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according...More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Human or rat P2X7-1321N1 cells were collected and frozen @ −80° C. On the day of the experiment, cell membrane preparations were made according...More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t...More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Human or rat P2X7-1321 N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according...More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.30nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 minsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.30nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 minsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.5nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.5nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 minsMore data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t...More data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:human or rat P2X7-1321 N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according...More data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t...More data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Human or rat P2X7-1321N1 cells were collected and frozen @ −80° C. On the day of the experiment, cell membrane preparations were made according...More data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Human or rat P2X7-1321 N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according...More data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Human or rat P2X7-1321 N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Human or rat P2X7-1321N1 cells were collected and frozen @ −80° C. On the day of the experiment, cell membrane preparations were made according...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Receptor binding was performed using membrane fractions prepared from the HEK-293 cell line recombinantly expressing rat 5-HT7 receptors (NCBI access...More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 minsMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Receptor binding was performed using membrane fractions prepared from the HEK-293 cell line recombinantly expressing rat 5-HT7 receptors (NCBI access...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Receptor binding was performed using membrane fractions prepared from the HEK-293 cell line recombinantly expressing rat 5-HT7 receptors (NCBI access...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:human or rat P2X7-1321 N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according...More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 2.10nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t...More data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:Human or rat P2X7-1321 N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according...More data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:Human or rat P2X7-1321 N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according...More data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:human or rat P2X7-1321 N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according...More data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t...More data for this Ligand-Target Pair