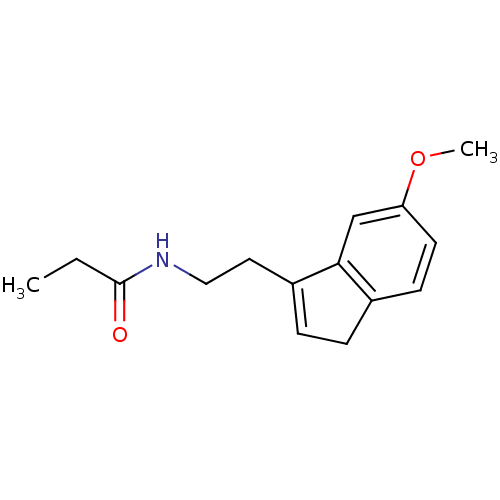
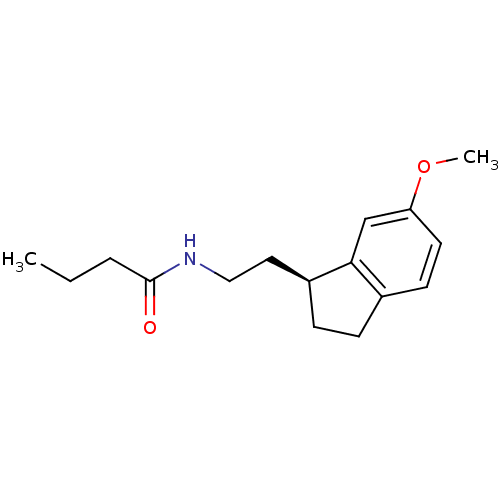
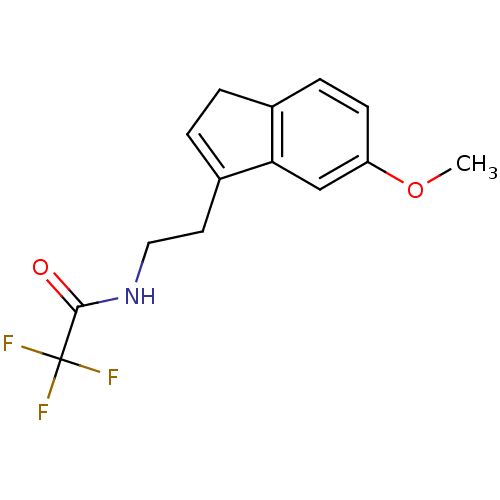
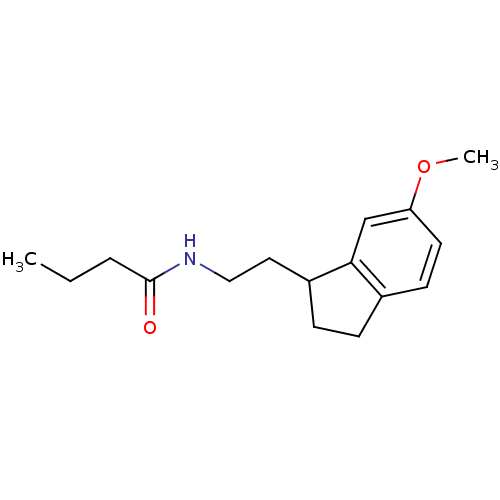
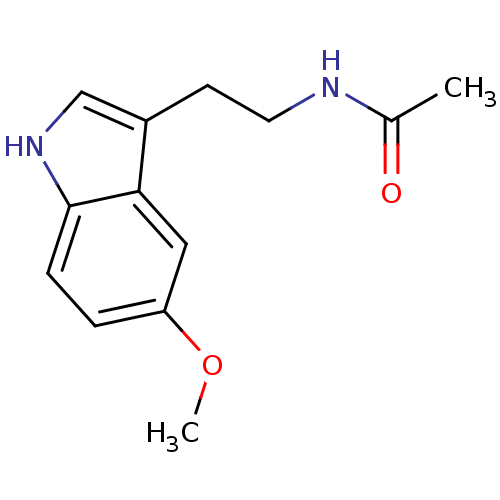
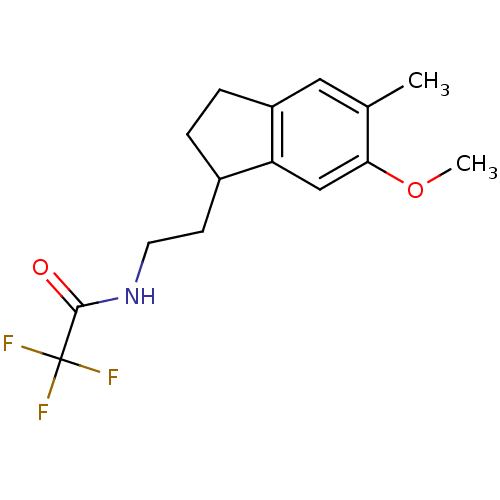
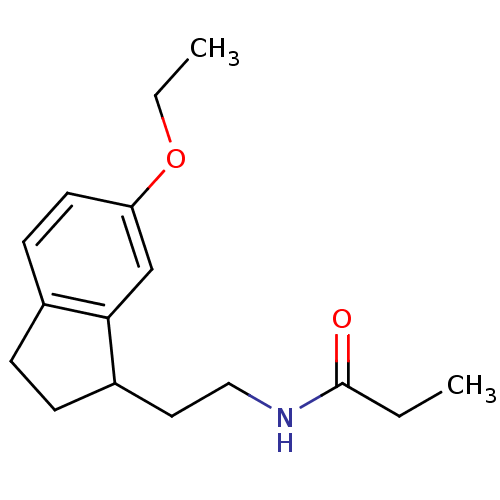
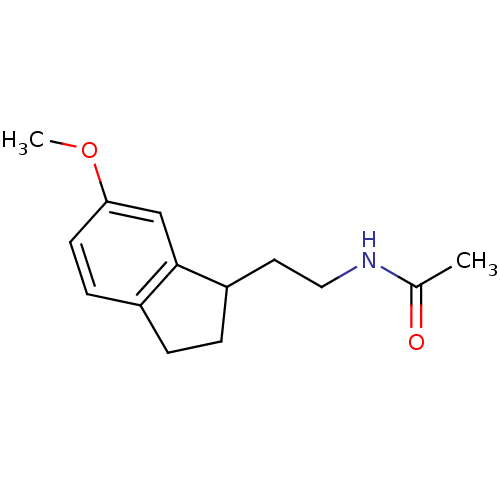
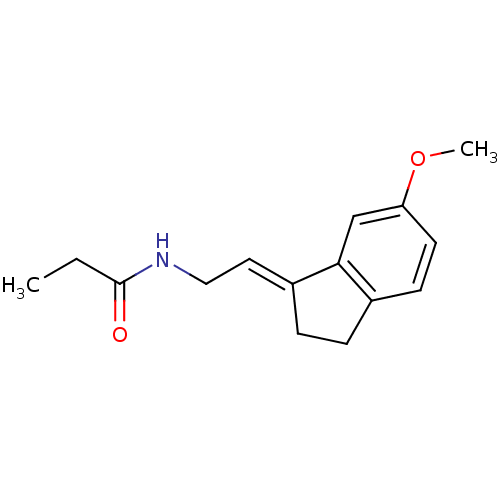
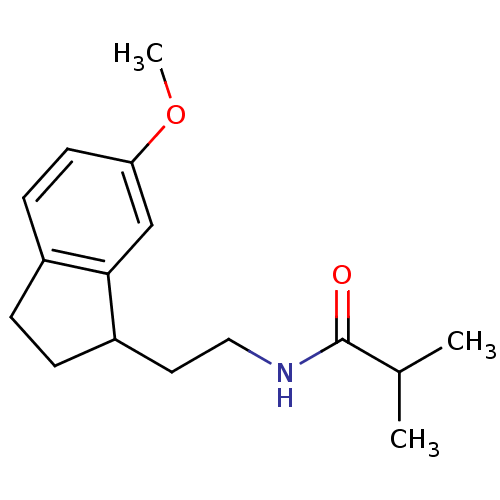
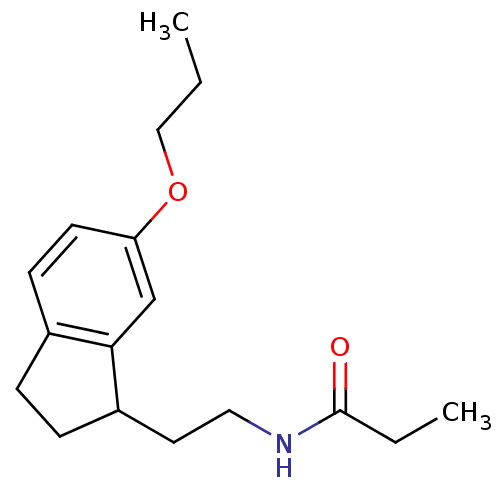
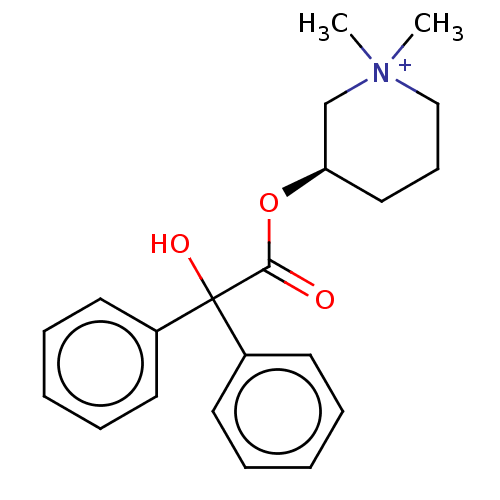
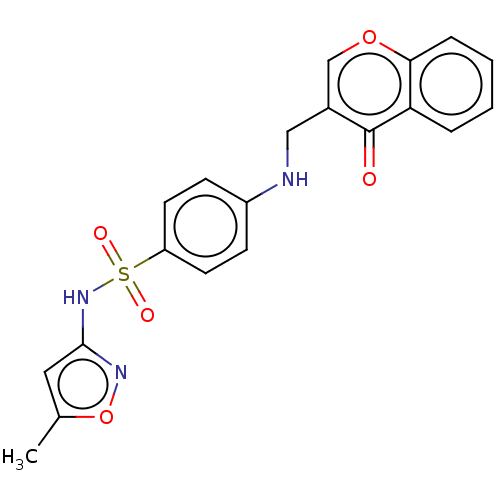
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 0.00602nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 0.0123nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
Affinity DataKi: 0.0138nMAssay Description:Binding affinity against human Melatonin receptor type 1A (MT1)More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 0.0225nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 0.0231nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 0.0321nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 0.0408nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 0.0410nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
Affinity DataKi: 0.0450nMAssay Description:Binding affinity against human Melatonin receptor type 1B (MT2)More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 0.0469nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 0.0553nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 0.0728nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 0.0733nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
Affinity DataKi: 0.0823nMAssay Description:Binding affinity against human Melatonin receptor type 1A (MT1)More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 0.0823nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 0.0984nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 0.100nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 0.131nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
Affinity DataKi: 0.195nMAssay Description:Binding affinity against human Melatonin receptor type 1B (MT2)More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 0.208nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 0.25nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 0.425nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
Affinity DataKi: 0.450nMAssay Description:Displacement of [3H]NMS from human M2R expressed in CHOK1 cells after 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 0.526nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
Affinity DataKi: 0.680nMAssay Description:Displacement of [3H]NMS from human M2R expressed in CHOK1 cells after 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 0.927nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Antagonist activity at human Dopamine receptor D2 isoform long expressed in HEK293 cells assessed as change in quinpirole-induced intracellular calci...More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 1.30nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 1.5nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 1.60nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 1.70nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
Affinity DataKi: 2.10nMAssay Description:Displacement of [3H]NMS from human M3R expressed in CHOK1 cells after 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.5nMAssay Description:Displacement of [3H]NMS from human M2R expressed in CHOK1 cells after 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.60nMAssay Description:Displacement of [3H]NMS from human M3R expressed in CHOK1 cells after 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 4.10nMAssay Description:Antagonist activity at human Dopamine receptor D2 isoform long expressed in HEK293 cells assessed as change in quinpirole-induced intracellular calci...More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 4.10nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
Affinity DataKi: 4.5nMAssay Description:Inhibition of human membrane associated form of carbonic anhydrase-12 assessed as CO2 hydration activity incubated for 15 mins prior to testing by st...More data for this Ligand-Target Pair
Affinity DataKi: 5.20nMAssay Description:Inhibition of human membrane associated form of carbonic anhydrase-12 assessed as CO2 hydration activity incubated for 15 mins prior to testing by st...More data for this Ligand-Target Pair
Affinity DataKi: 5.70nMAssay Description:Inhibition of human membrane associated form of carbonic anhydrase-12 assessed as CO2 hydration activity incubated for 15 mins prior to testing by st...More data for this Ligand-Target Pair
Affinity DataKi: 6.20nMAssay Description:Antagonist activity at human Dopamine receptor D2 isoform long expressed in HEK293 cells assessed as change in quinpirole-induced intracellular calci...More data for this Ligand-Target Pair
Affinity DataKi: 6.20nMAssay Description:Inhibition of human membrane associated form of carbonic anhydrase-12 assessed as CO2 hydration activity incubated for 15 mins prior to testing by st...More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 6.40nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 6.80nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
TargetRibosyldihydronicotinamide dehydrogenase [quinone](Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 9.10nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to melatonin receptor 3 (MT3) of Syrian hamster brain.More data for this Ligand-Target Pair
Affinity DataKi: 9.70nMAssay Description:Inhibition of human membrane associated form of carbonic anhydrase-12 assessed as CO2 hydration activity incubated for 15 mins prior to testing by st...More data for this Ligand-Target Pair
Affinity DataKi: 9.90nMAssay Description:Inhibition of human membrane associated form of carbonic anhydrase-12 assessed as CO2 hydration activity incubated for 15 mins prior to testing by st...More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Inhibition of human membrane associated form of carbonic anhydrase-12 assessed as CO2 hydration activity incubated for 15 mins prior to testing by st...More data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Inhibition of human cytosolic form of carbonic anhydrase-2 assessed as CO2 hydration activity incubated for 15 mins prior to testing by stopped flow ...More data for this Ligand-Target Pair
TargetMelatonin receptor type 1A/1B(Homo sapiens (Human))
Takeda Chemical Industries
Curated by ChEMBL
Takeda Chemical Industries
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.More data for this Ligand-Target Pair

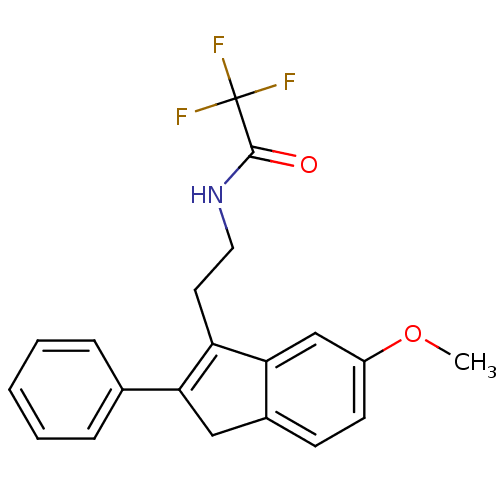
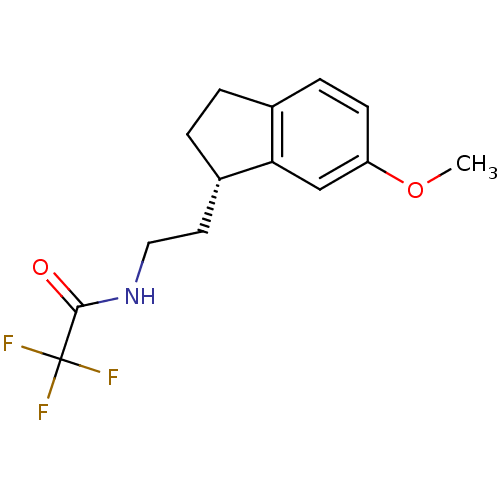

 3D Structure (crystal)
3D Structure (crystal)