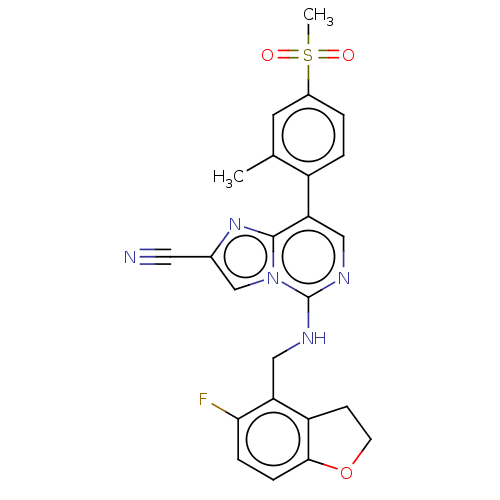
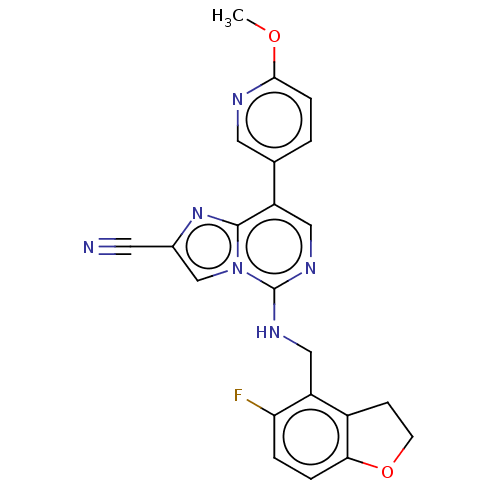
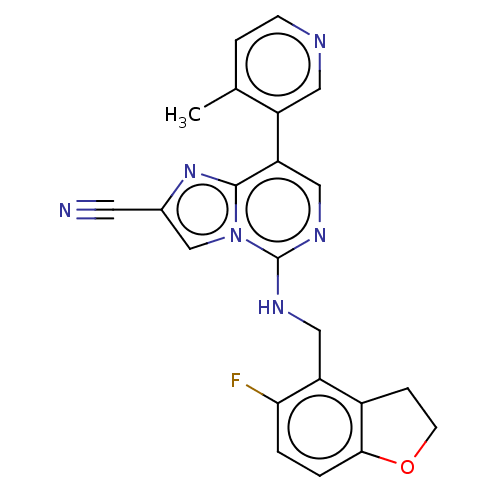
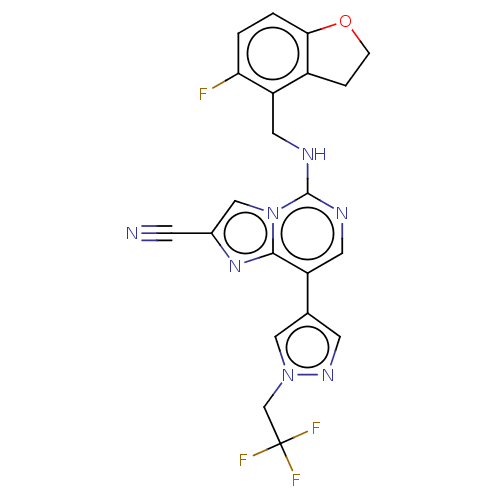
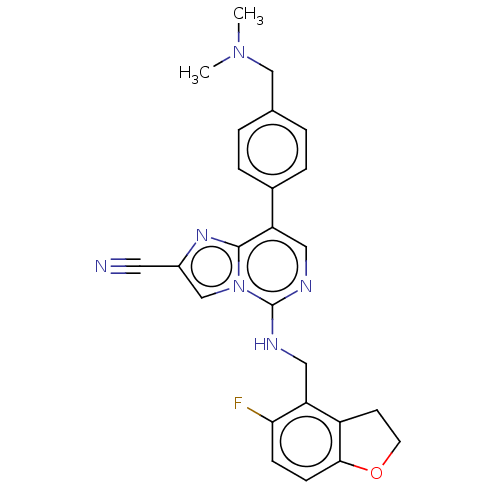
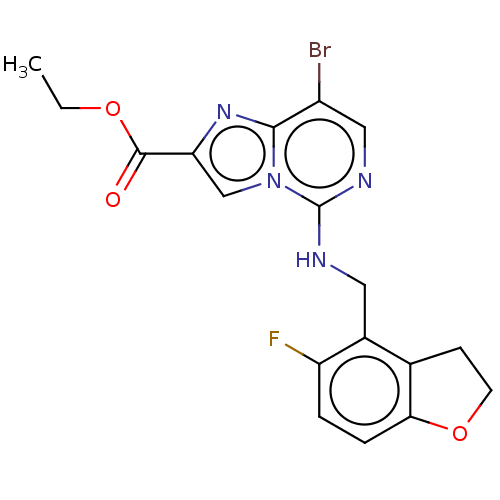
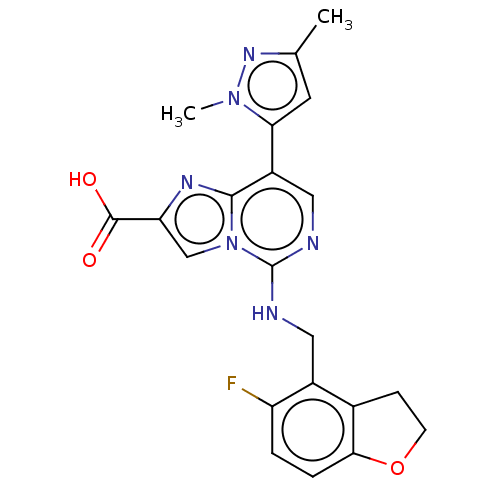
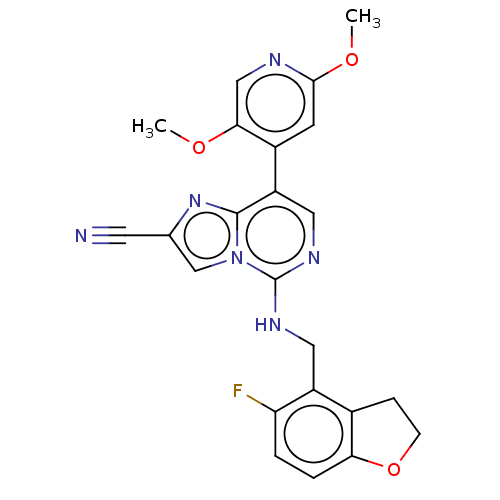
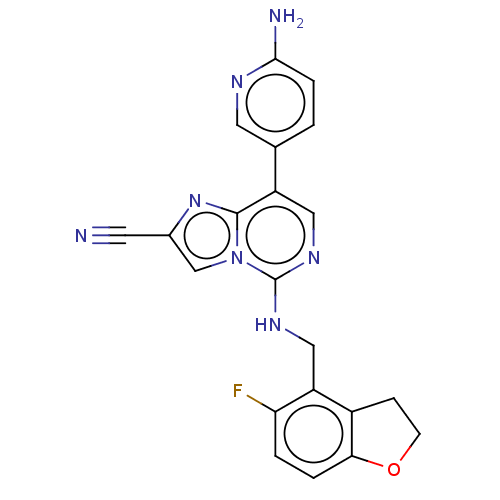
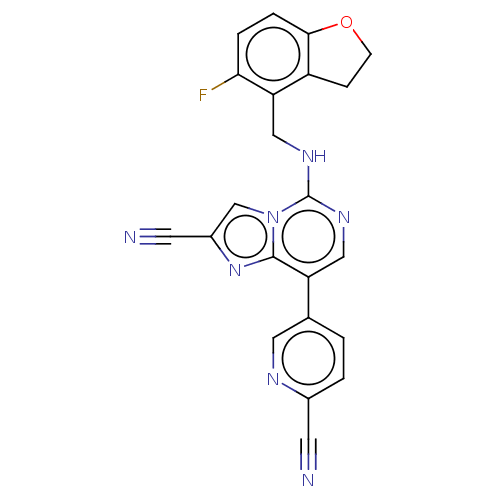
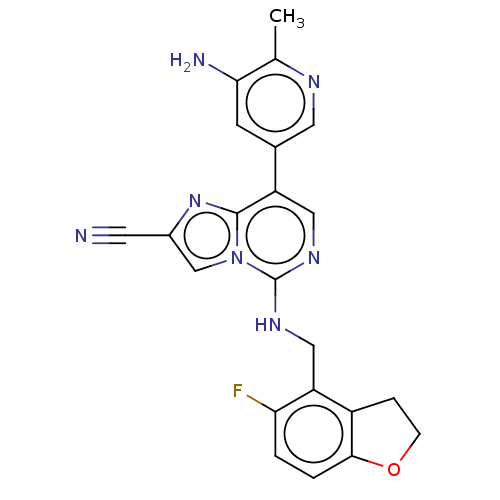
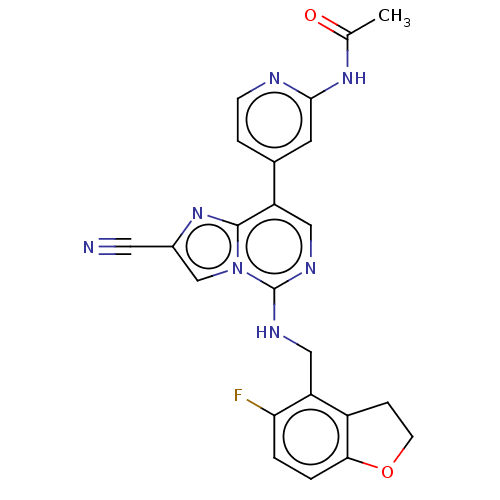
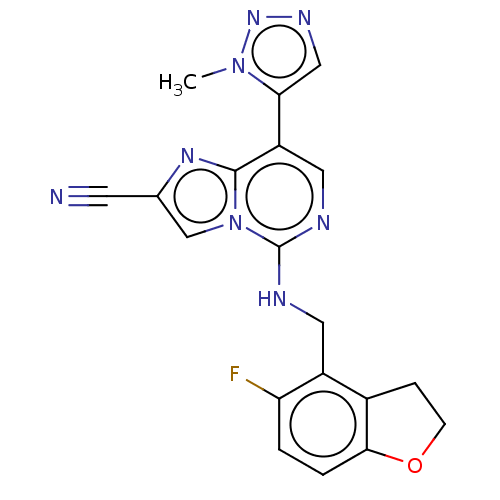
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
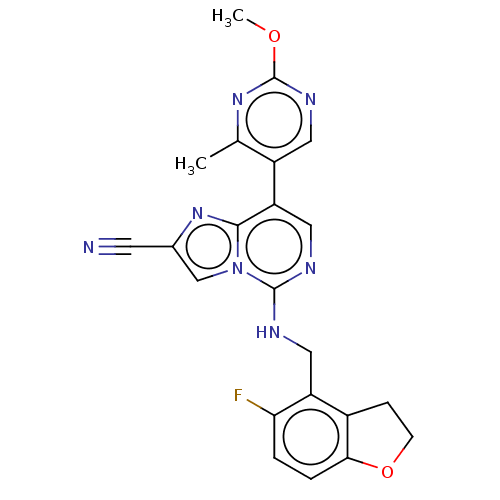
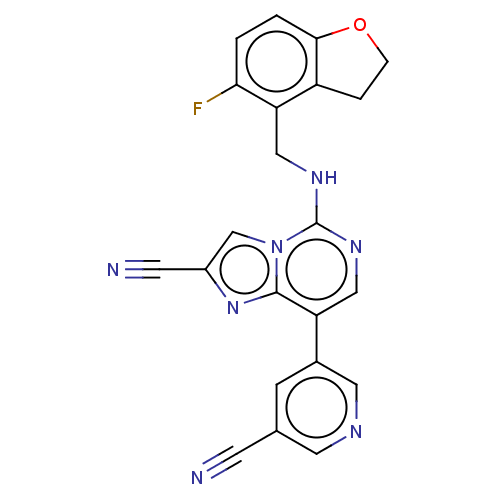
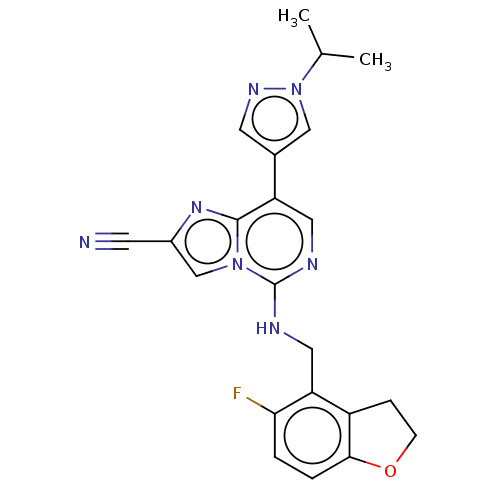
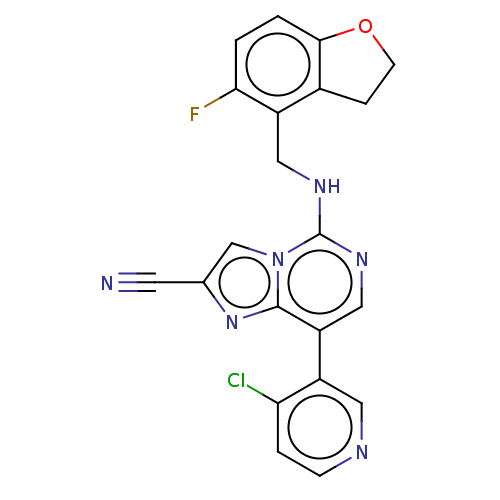
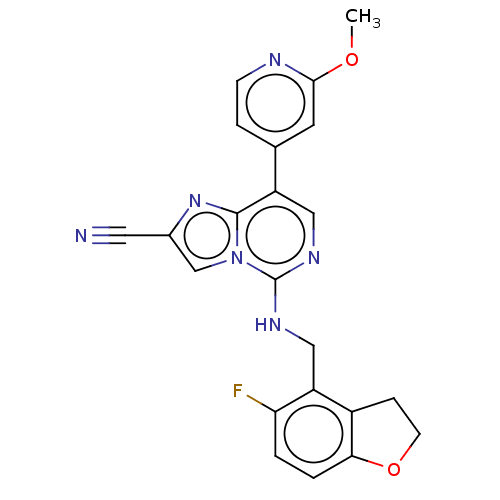
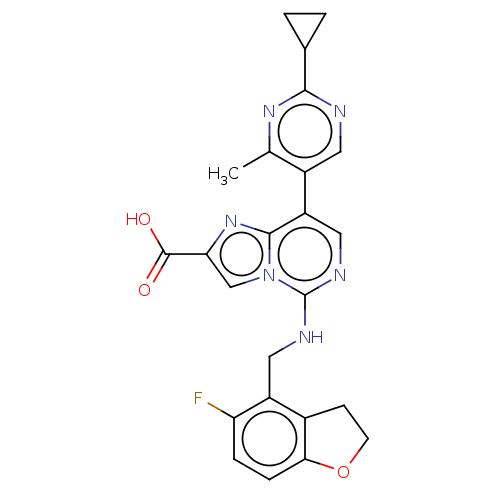
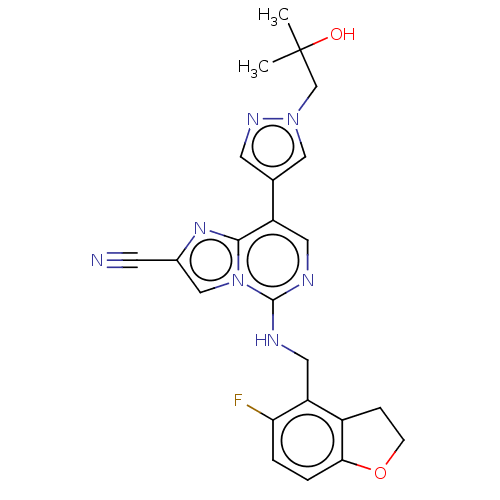
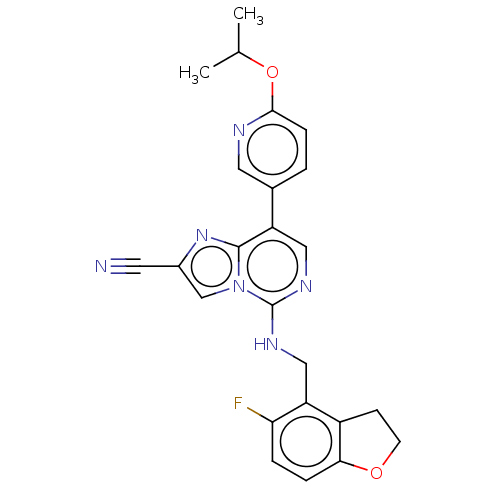
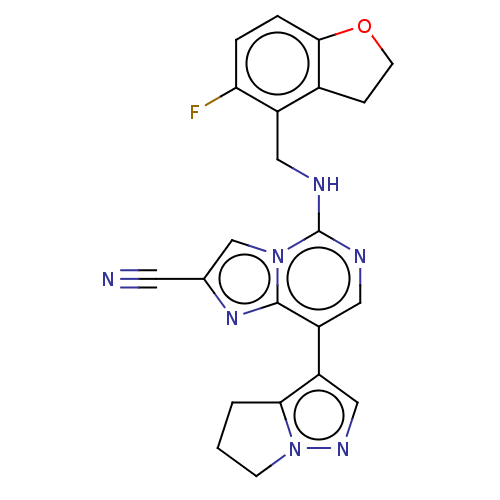
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
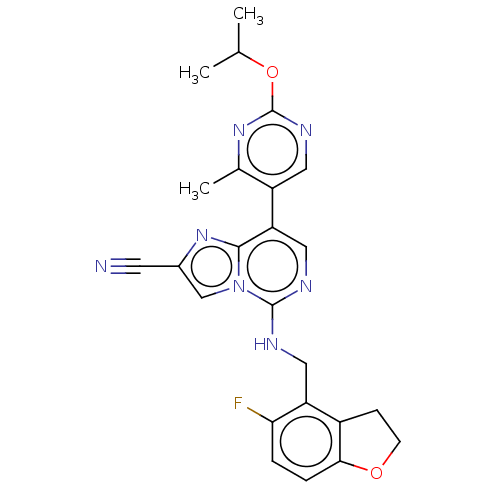
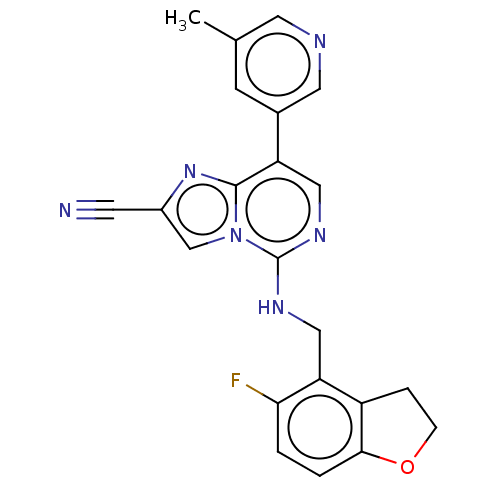
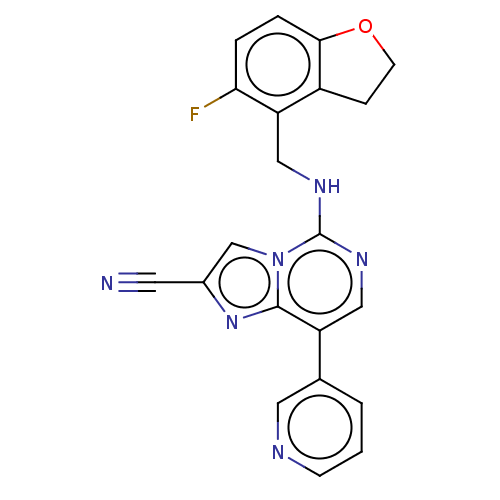
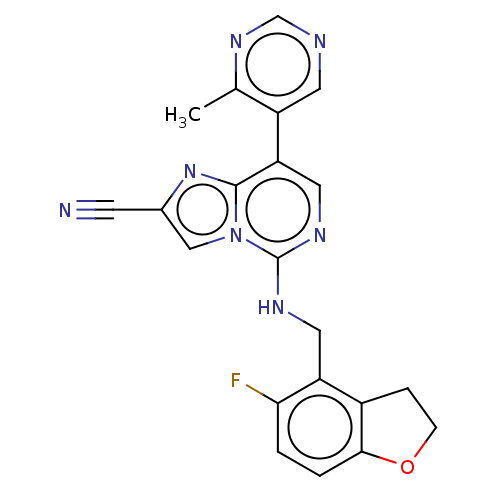
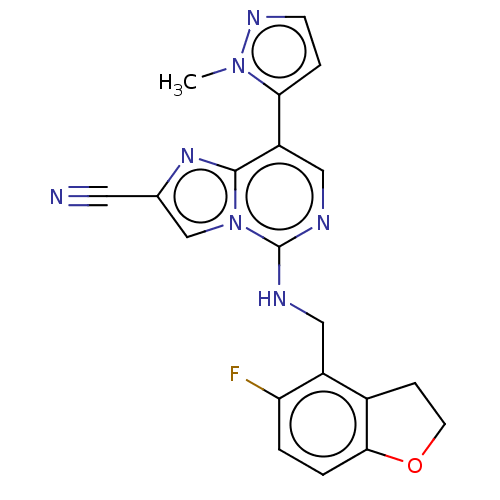
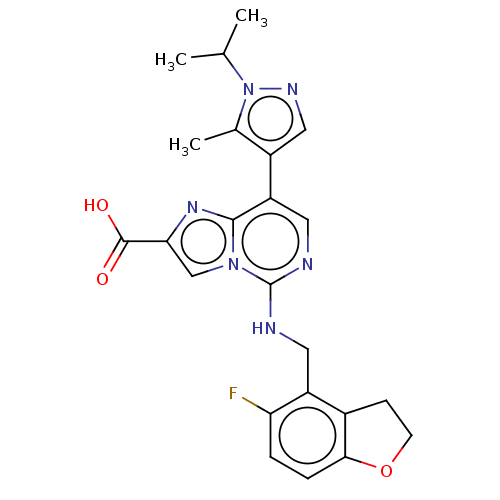
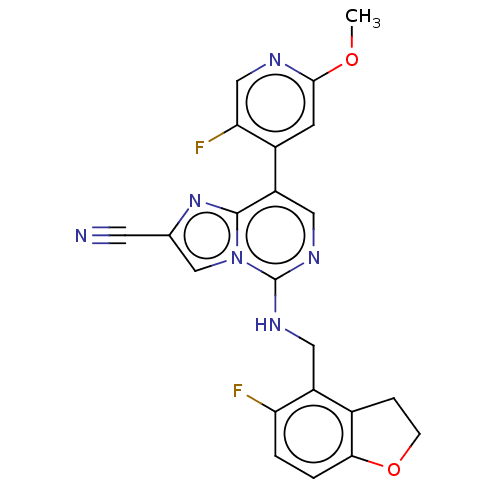
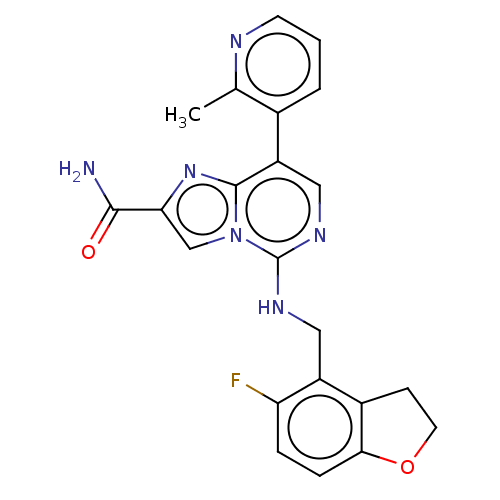
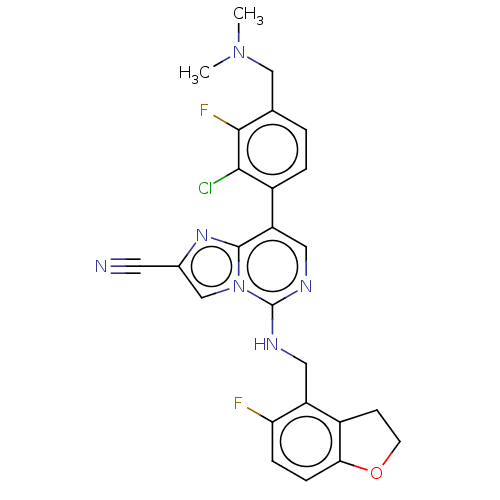
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
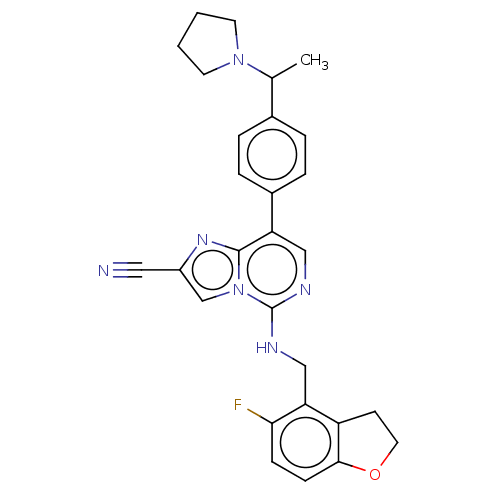
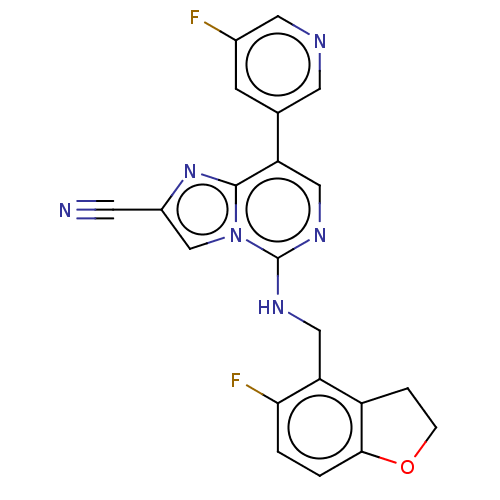
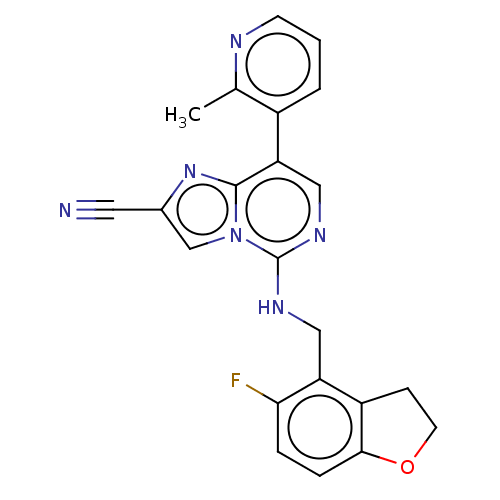
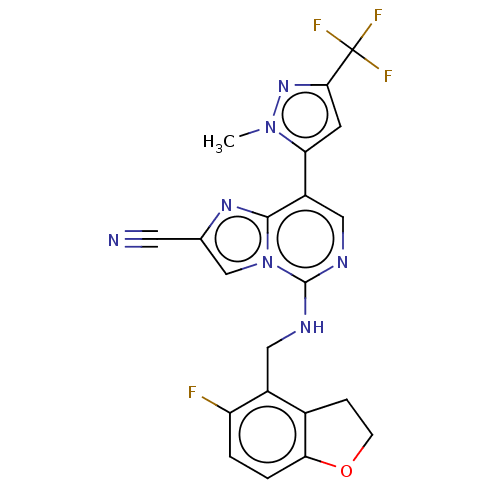
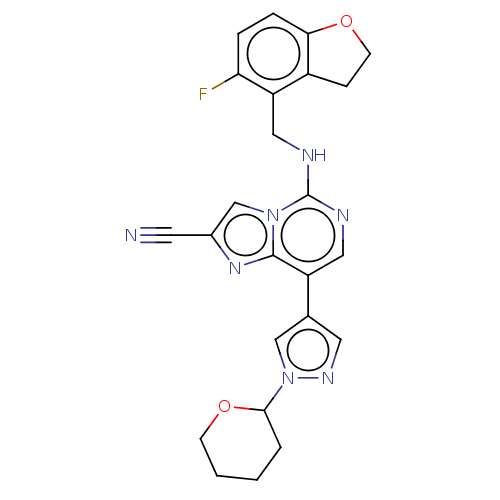
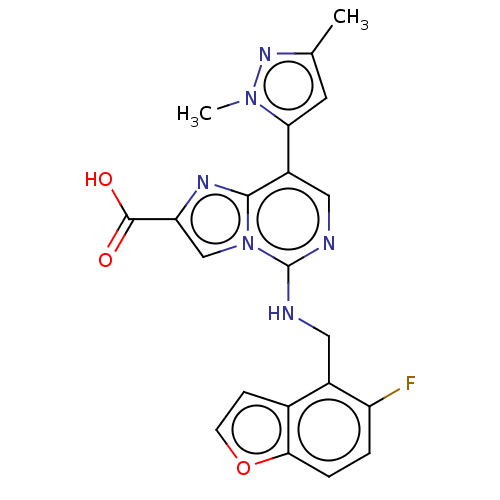
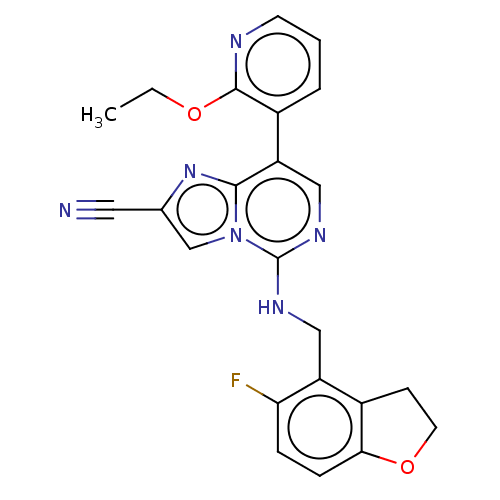
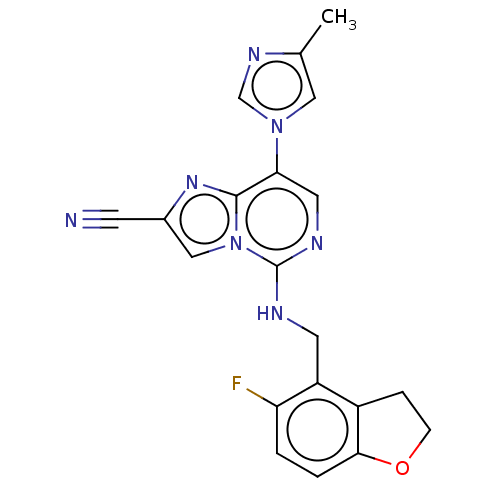
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Homo sapiens (Human))
Mirati Therapeutics
US Patent
Mirati Therapeutics
US Patent
Affinity DataIC50: <100nMAssay Description:Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1...More data for this Ligand-Target Pair



 3D Structure (crystal)
3D Structure (crystal)