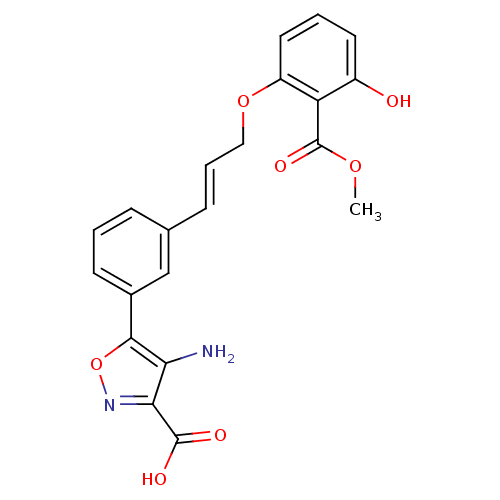
Affinity DataKi: 2.10nMAssay Description:Inhibition of PTP1B (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1 [1-298](Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 2.10E+3nM ΔG°: -32.1kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 2.10E+3nMAssay Description:Inhibition of PTP1BMore data for this Ligand-Target Pair
Affinity DataKi: 1.58E+4nMAssay Description:Inhibition of PTP1BMore data for this Ligand-Target Pair
Affinity DataKi: >3.00E+4nM ΔG°: >-25.6kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair