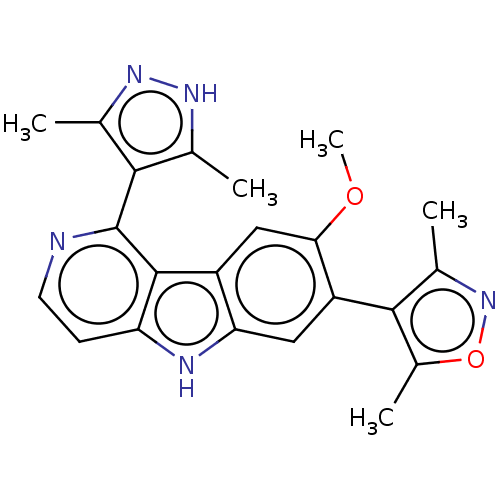
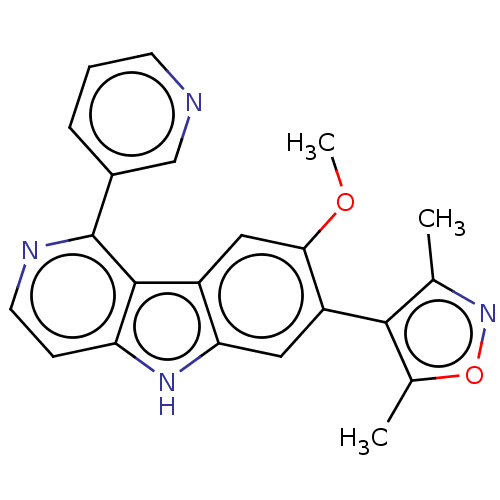
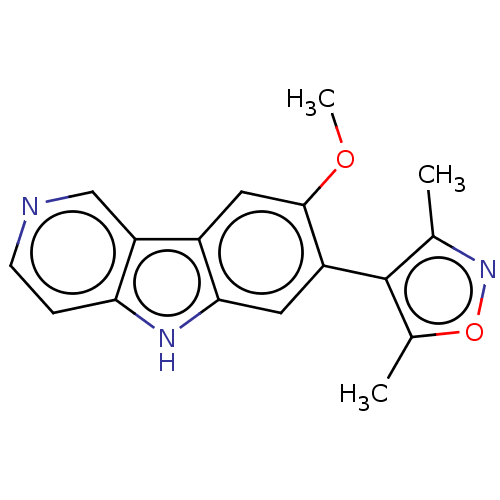
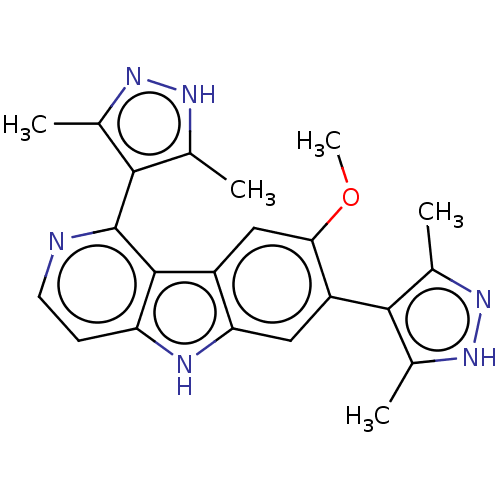
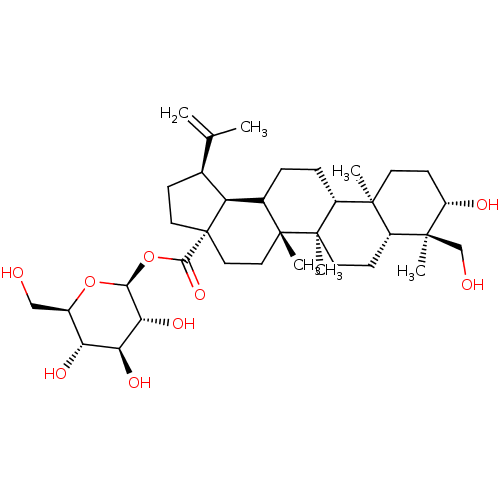
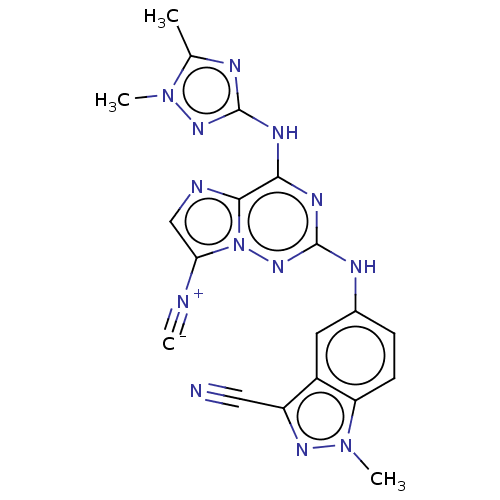
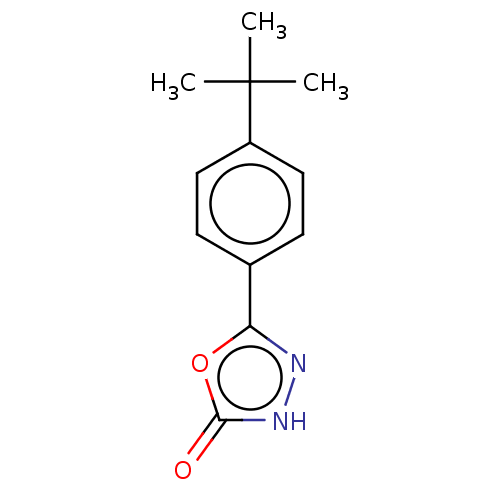
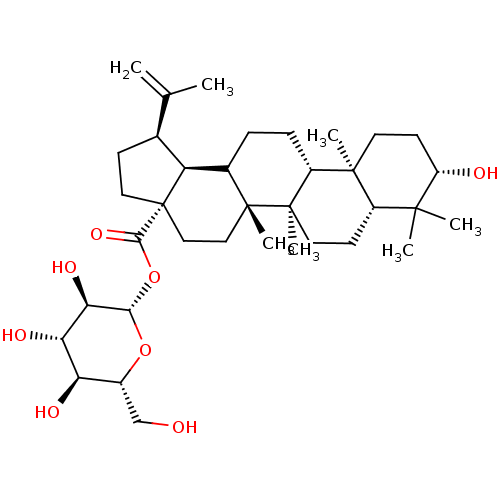
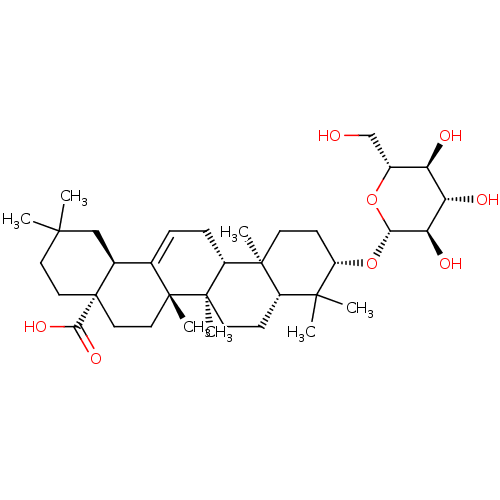
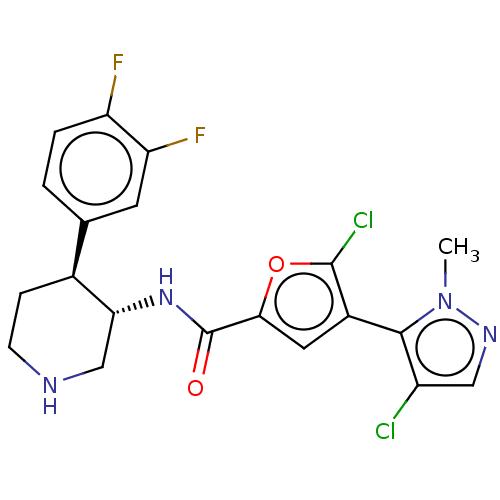
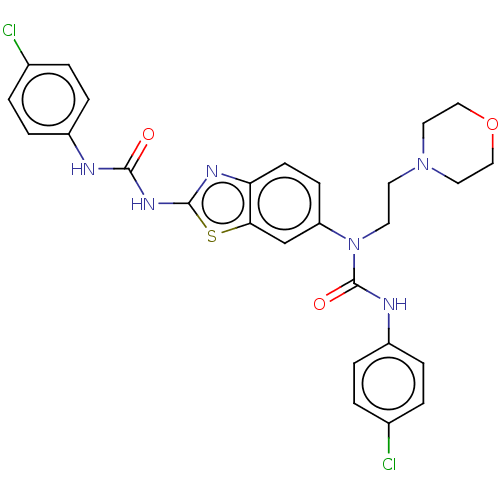
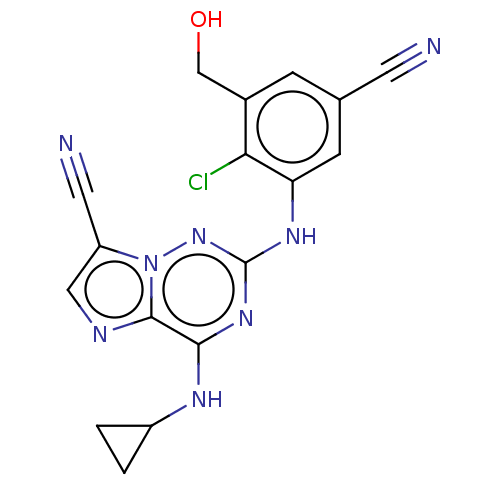
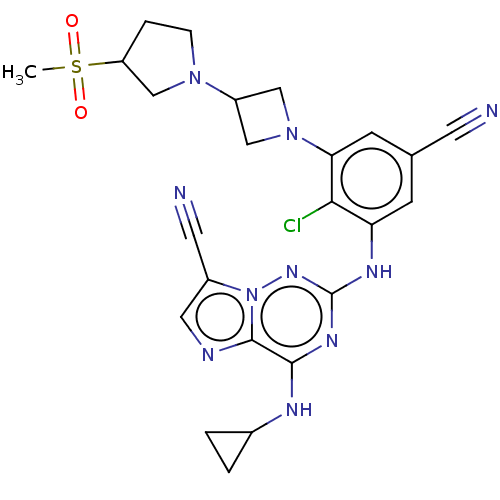
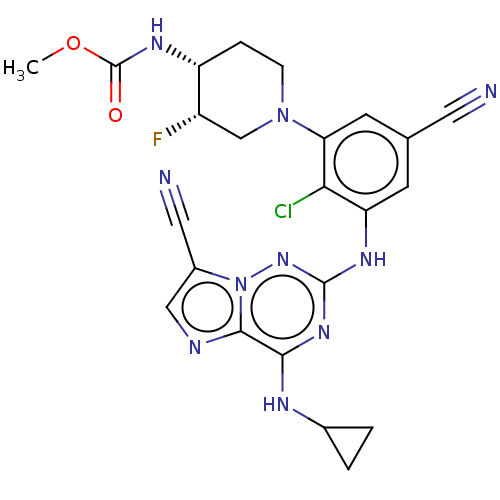
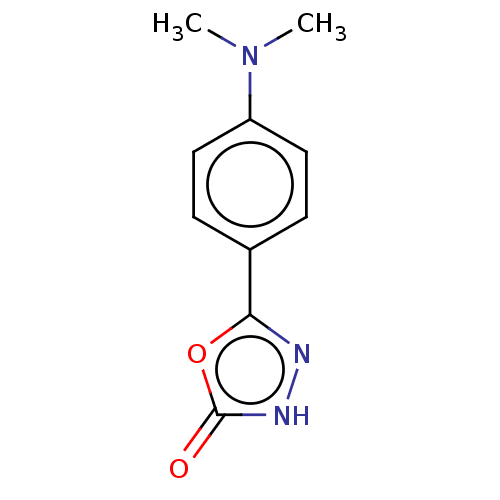
TargetBromodomain-containing protein 2 [364-436](Homo sapiens (Human))
The Regents of The University of Michigan
US Patent
The Regents of The University of Michigan
US Patent
Affinity DataKi: <10nM ΔG°: <-45.7kJ/mole IC50: 93nMpH: 7.5 T: 2°CAssay Description:The FAM labeled fluorescent probe (BRD-1F) was synthesized based on a known small-molecule BET bromodomain inhibitor. Kd values of BRD-1F to these th...More data for this Ligand-Target Pair
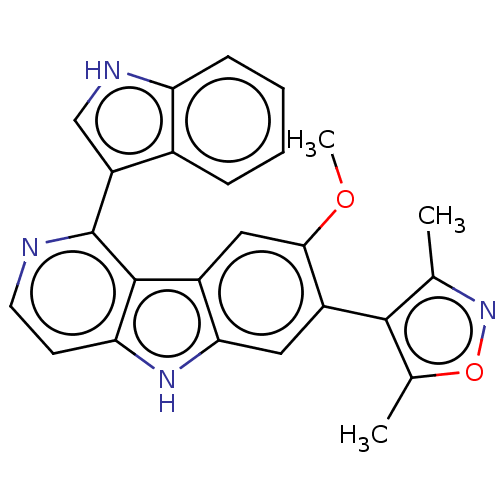
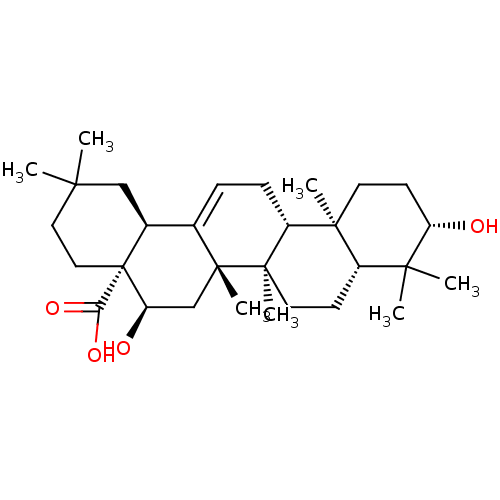
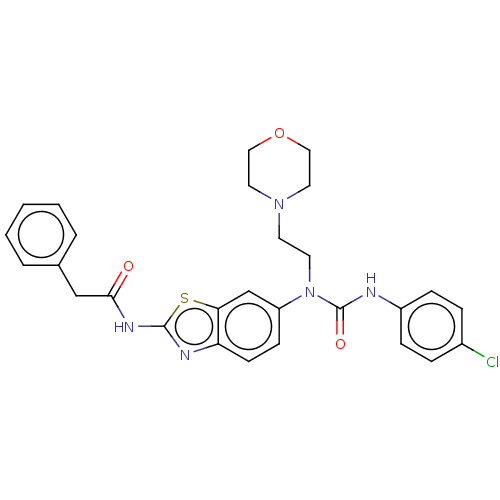
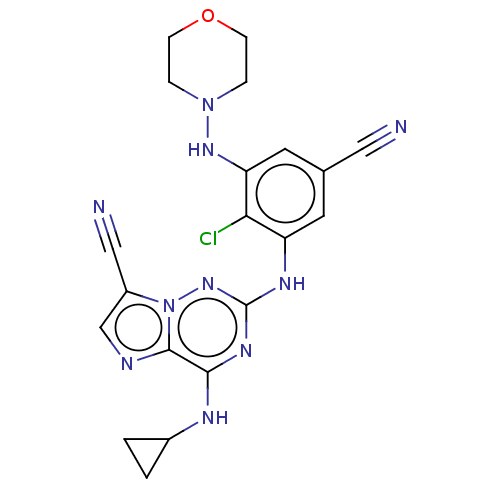
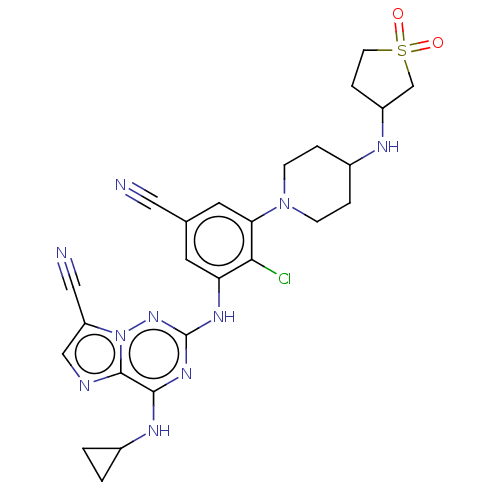
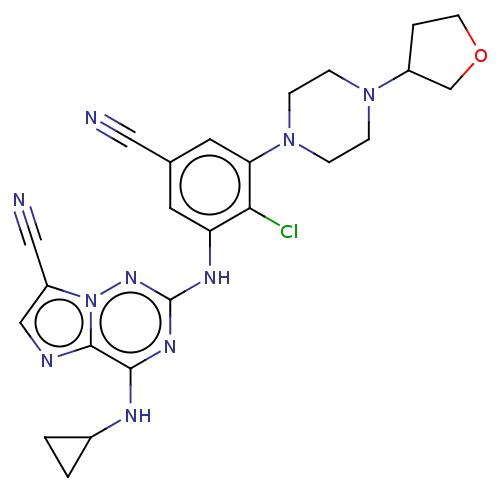
TargetBromodomain-containing protein 2 [364-436](Homo sapiens (Human))
The Regents of The University of Michigan
US Patent
The Regents of The University of Michigan
US Patent
Affinity DataKi: <10nM ΔG°: <-45.7kJ/mole IC50: 142nMpH: 7.5 T: 2°CAssay Description:The FAM labeled fluorescent probe (BRD-1F) was synthesized based on a known small-molecule BET bromodomain inhibitor. Kd values of BRD-1F to these th...More data for this Ligand-Target Pair
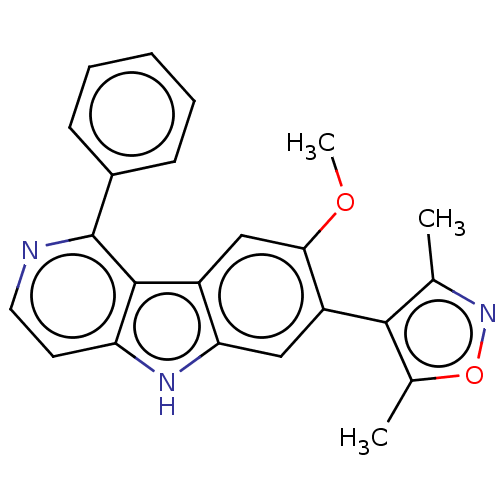
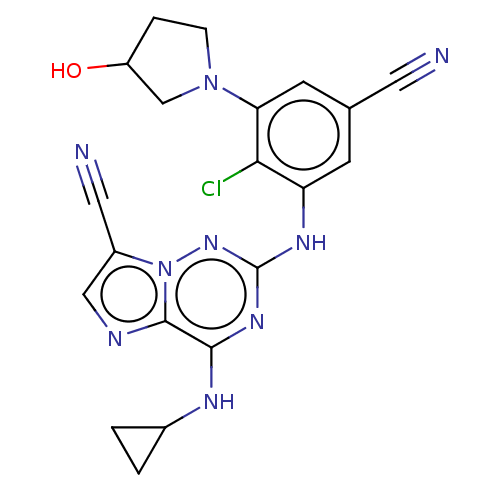
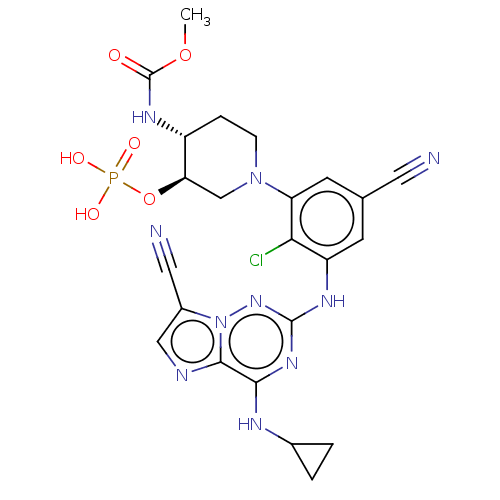
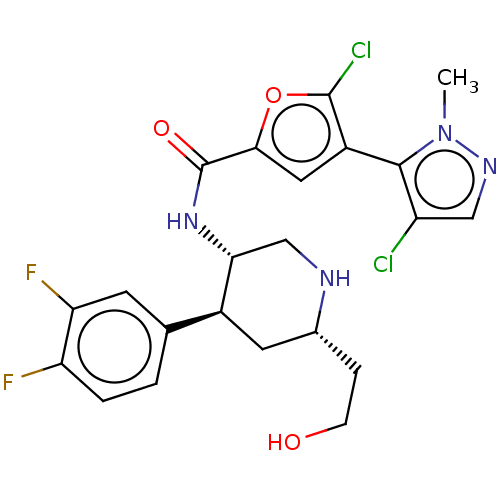
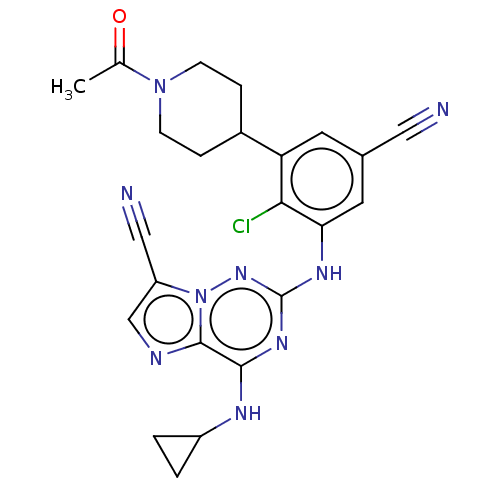
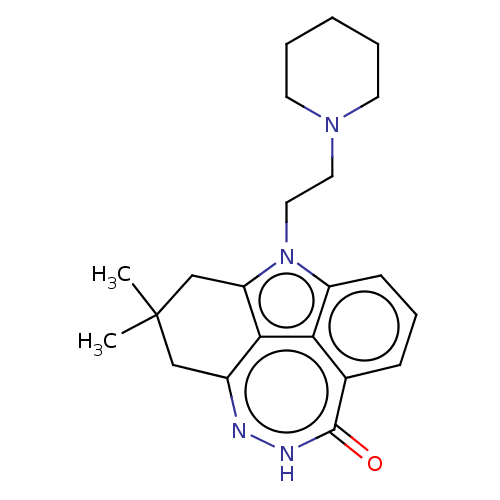
TargetBromodomain-containing protein 4 [368-440](Homo sapiens (Human))
The Regents of The University of Michigan
US Patent
The Regents of The University of Michigan
US Patent
Affinity DataKi: 16nM ΔG°: -44.5kJ/mole IC50: 197nMpH: 7.5 T: 2°CAssay Description:The FAM labeled fluorescent probe (BRD-1F) was synthesized based on a known small-molecule BET bromodomain inhibitor. Kd values of BRD-1F to these th...More data for this Ligand-Target Pair
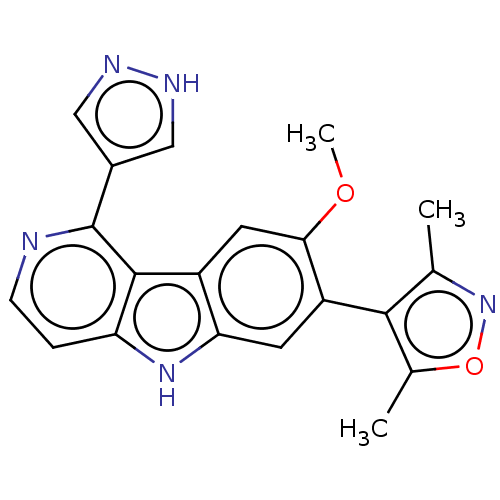
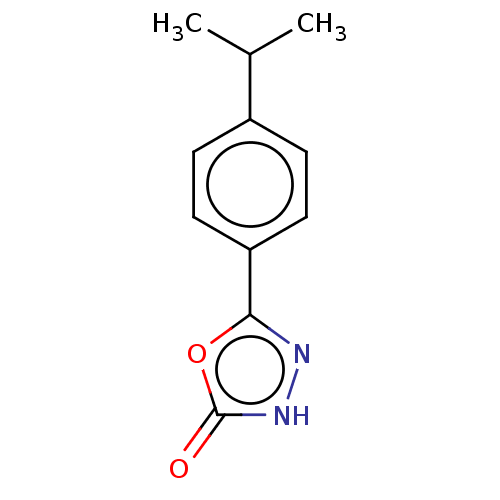
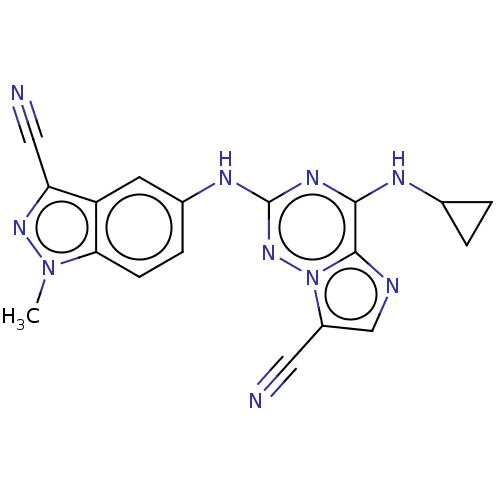
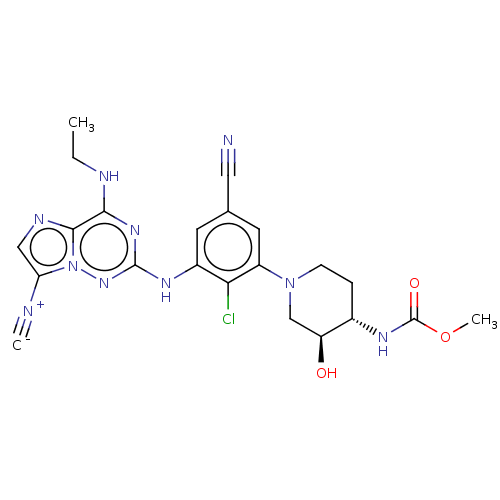
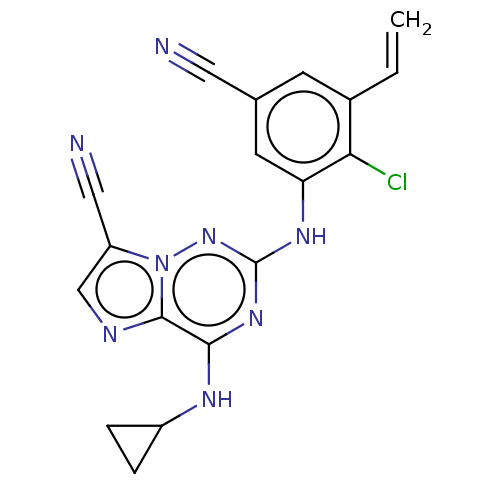
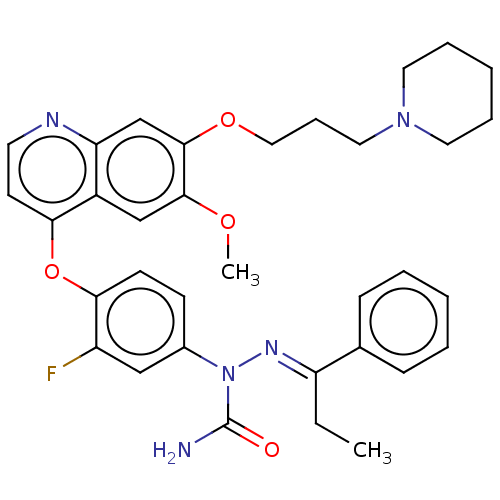
TargetBromodomain-containing protein 2 [364-436](Homo sapiens (Human))
The Regents of The University of Michigan
US Patent
The Regents of The University of Michigan
US Patent
Affinity DataKi: 35nM ΔG°: -42.6kJ/mole IC50: 255nMpH: 7.5 T: 2°CAssay Description:The FAM labeled fluorescent probe (BRD-1F) was synthesized based on a known small-molecule BET bromodomain inhibitor. Kd values of BRD-1F to these th...More data for this Ligand-Target Pair
TargetBromodomain-containing protein 4 [368-440](Homo sapiens (Human))
The Regents of The University of Michigan
US Patent
The Regents of The University of Michigan
US Patent
Affinity DataKi: 38nM ΔG°: -42.4kJ/mole IC50: 310nMpH: 7.5 T: 2°CAssay Description:The FAM labeled fluorescent probe (BRD-1F) was synthesized based on a known small-molecule BET bromodomain inhibitor. Kd values of BRD-1F to these th...More data for this Ligand-Target Pair
TargetBromodomain-containing protein 2 [364-436](Homo sapiens (Human))
The Regents of The University of Michigan
US Patent
The Regents of The University of Michigan
US Patent
Affinity DataKi: 42.3nM ΔG°: -42.1kJ/mole IC50: 286nMpH: 7.5 T: 2°CAssay Description:The FAM labeled fluorescent probe (BRD-1F) was synthesized based on a known small-molecule BET bromodomain inhibitor. Kd values of BRD-1F to these th...More data for this Ligand-Target Pair
TargetBromodomain-containing protein 2 [364-436](Homo sapiens (Human))
The Regents of The University of Michigan
US Patent
The Regents of The University of Michigan
US Patent
Affinity DataKi: 57nM ΔG°: -41.3kJ/mole IC50: 349nMpH: 7.5 T: 2°CAssay Description:The FAM labeled fluorescent probe (BRD-1F) was synthesized based on a known small-molecule BET bromodomain inhibitor. Kd values of BRD-1F to these th...More data for this Ligand-Target Pair
TargetBromodomain-containing protein 4 [368-440](Homo sapiens (Human))
The Regents of The University of Michigan
US Patent
The Regents of The University of Michigan
US Patent
Affinity DataKi: 83nM ΔG°: -40.4kJ/mole IC50: 514nMpH: 7.5 T: 2°CAssay Description:The FAM labeled fluorescent probe (BRD-1F) was synthesized based on a known small-molecule BET bromodomain inhibitor. Kd values of BRD-1F to these th...More data for this Ligand-Target Pair
TargetBromodomain-containing protein 4 [368-440](Homo sapiens (Human))
The Regents of The University of Michigan
US Patent
The Regents of The University of Michigan
US Patent
Affinity DataKi: 114nM ΔG°: -39.6kJ/mole IC50: 608nMpH: 7.5 T: 2°CAssay Description:The FAM labeled fluorescent probe (BRD-1F) was synthesized based on a known small-molecule BET bromodomain inhibitor. Kd values of BRD-1F to these th...More data for this Ligand-Target Pair
TargetBromodomain-containing protein 4 [368-440](Homo sapiens (Human))
The Regents of The University of Michigan
US Patent
The Regents of The University of Michigan
US Patent
Affinity DataKi: 232nM ΔG°: -37.9kJ/mole IC50: 873nMpH: 7.5 T: 2°CAssay Description:The FAM labeled fluorescent probe (BRD-1F) was synthesized based on a known small-molecule BET bromodomain inhibitor. Kd values of BRD-1F to these th...More data for this Ligand-Target Pair
TargetBromodomain-containing protein 2 [364-436](Homo sapiens (Human))
The Regents of The University of Michigan
US Patent
The Regents of The University of Michigan
US Patent
Affinity DataKi: 366nM ΔG°: -36.7kJ/mole IC50: 1.35E+3nMpH: 7.5 T: 2°CAssay Description:The FAM labeled fluorescent probe (BRD-1F) was synthesized based on a known small-molecule BET bromodomain inhibitor. Kd values of BRD-1F to these th...More data for this Ligand-Target Pair
TargetBromodomain-containing protein 4 [368-440](Homo sapiens (Human))
The Regents of The University of Michigan
US Patent
The Regents of The University of Michigan
US Patent
Affinity DataKi: 775nM ΔG°: -34.9kJ/mole IC50: 3.67E+3nMpH: 7.5 T: 2°CAssay Description:The FAM labeled fluorescent probe (BRD-1F) was synthesized based on a known small-molecule BET bromodomain inhibitor. Kd values of BRD-1F to these th...More data for this Ligand-Target Pair
TargetBromodomain-containing protein 4 [368-440](Homo sapiens (Human))
The Regents of The University of Michigan
US Patent
The Regents of The University of Michigan
US Patent
Affinity DataKi: 850nM ΔG°: -34.6kJ/mole IC50: 2.90E+3nMpH: 7.5 T: 2°CAssay Description:The FAM labeled fluorescent probe (BRD-1F) was synthesized based on a known small-molecule BET bromodomain inhibitor. Kd values of BRD-1F to these th...More data for this Ligand-Target Pair
TargetBromodomain-containing protein 2 [364-436](Homo sapiens (Human))
The Regents of The University of Michigan
US Patent
The Regents of The University of Michigan
US Patent
Affinity DataKi: 1.20E+3nM ΔG°: -33.8kJ/mole IC50: 3.20E+3nMpH: 7.5 T: 2°CAssay Description:The FAM labeled fluorescent probe (BRD-1F) was synthesized based on a known small-molecule BET bromodomain inhibitor. Kd values of BRD-1F to these th...More data for this Ligand-Target Pair
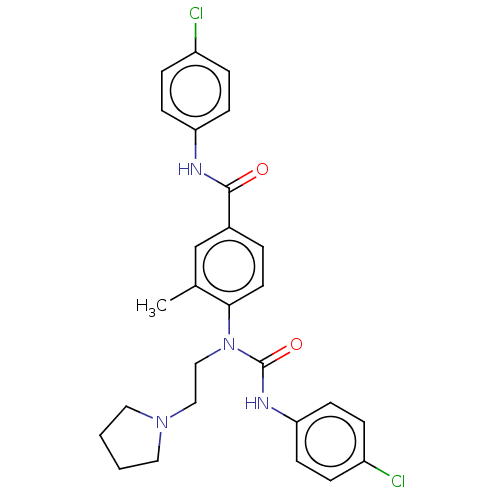
Affinity DataIC50: 0.0280nMAssay Description:Inhibition of LPS-induced tissue factor procoagulant activity in human THP1 cells preincubated for 1 hr followed by LPS addition measured after 5 hrs...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0300nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Shenyang Pharmaceutical University
Curated by ChEMBL
Shenyang Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 0.0300nMAssay Description:Inhibition of sEH (unknown origin)More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Hangzhou Xixi Hospital
Curated by ChEMBL
Hangzhou Xixi Hospital
Curated by ChEMBL
Affinity DataIC50: 0.0350nMAssay Description:Inhibition of VEGFR-2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.0350nMAssay Description:Inhibition of LPS-induced tissue factor procoagulant activity in human THP1 cells preincubated for 1 hr followed by LPS addition measured after 5 hrs...More data for this Ligand-Target Pair
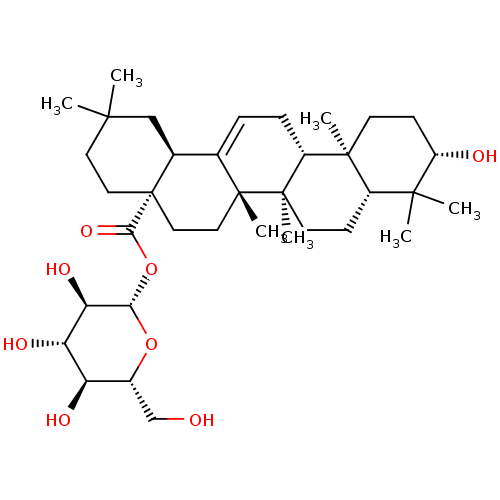
TargetPalmitoleoyl-protein carboxylesterase NOTUM(Homo sapiens (Human))
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
Affinity DataIC50: 0.0510nMAssay Description:Inhibition of human Notum (S81-T451 residues) C330S mutant expressed in HEK293S cells using OPTS substrate incubated for 40 mins by fluorescence base...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0540nMAssay Description:Inhibition of LPS-induced tissue factor procoagulant activity in human THP1 cells preincubated for 1 hr followed by LPS addition measured after 5 hrs...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0700nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
TargetPalmitoleoyl-protein carboxylesterase NOTUM(Homo sapiens (Human))
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
Affinity DataIC50: 0.0750nMAssay Description:Inhibition of human Notum (S81-T451 residues) C330S mutant expressed in HEK293S cells using OPTS substrate incubated for 40 mins by fluorescence base...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0780nMAssay Description:Inhibition of LPS-induced tissue factor procoagulant activity in human THP1 cells preincubated for 1 hr followed by LPS addition measured after 5 hrs...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Shenyang Pharmaceutical University
Curated by ChEMBL
Shenyang Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 0.0820nMAssay Description:Inhibition of recombinant human sEH using PHOME as substrate measured after 15 mins by fluorescence assayMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Hangzhou Xixi Hospital
Curated by ChEMBL
Hangzhou Xixi Hospital
Curated by ChEMBL
Affinity DataIC50: 0.0860nMAssay Description:Inhibition of VEGFR-2 (unknown origin) by HTRF methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0900nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0900nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0950nMAssay Description:Inhibition of LPS-induced tissue factor procoagulant activity in human THP1 cells preincubated for 1 hr followed by LPS addition measured after 5 hrs...More data for this Ligand-Target Pair
TargetE3 ubiquitin-protein ligase Mdm2(Homo sapiens (Human))
Shanghai Institute Of Materia Medica
Curated by ChEMBL
Shanghai Institute Of Materia Medica
Curated by ChEMBL
Affinity DataIC50: <0.100nMAssay Description:Inhibition of human MDM2 by TR-FRET assayMore data for this Ligand-Target Pair
TargetRAC-gamma serine/threonine-protein kinase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Inhibition of Akt3 (unknown origin) by mobile shift assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Shenyang Pharmaceutical University
Curated by ChEMBL
Shenyang Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Inhibition of recombinant human sEH using PHOME as substrate measured after 15 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.110nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.110nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.110nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
TargetRAC-gamma serine/threonine-protein kinase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 0.120nMAssay Description:Inhibition of Akt3 (unknown origin) by mobile shift assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.130nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.130nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.140nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.150nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.150nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.170nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
TargetPalmitoleoyl-protein carboxylesterase NOTUM(Homo sapiens (Human))
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
Affinity DataIC50: 0.190nMAssay Description:Inhibition of human Notum (S81-T451 residues) C330S mutant expressed in HEK293S cells using OPTS substrate incubated for 40 mins by fluorescence base...More data for this Ligand-Target Pair
TargetPalmitoleoyl-protein carboxylesterase NOTUM(Homo sapiens (Human))
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
Affinity DataIC50: 0.190nMAssay Description:Inhibition of human Notum (S81-T451 residues) C330S mutant expressed in HEK293S cells using OPTS substrate incubated for 40 mins by fluorescence base...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMpH: 7.4 T: 2°CAssay Description:The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibition of PARP-2 (unknown origin) pre-incubated for 30 mins before addition of activated DNA and NAD by chemiluminescent assayMore data for this Ligand-Target Pair
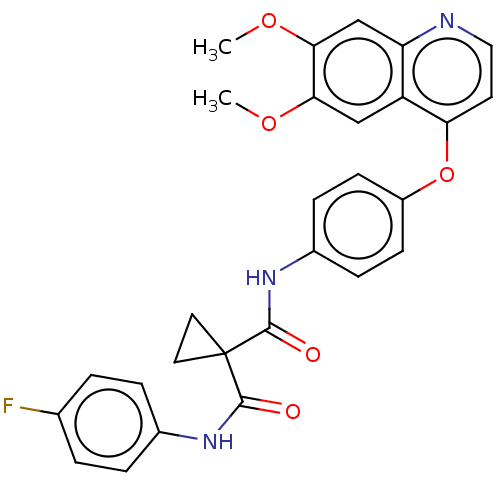
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Shenyang Pharmaceutical University
US Patent
Shenyang Pharmaceutical University
US Patent
Affinity DataIC50: 0.200nMAssay Description:c-Met Kinase Activity was Measured with an ELISA Reader. The Specific Operation as Follows:To the plate filled with 0.25 mg/mL PGT, the compounds, 50...More data for this Ligand-Target Pair