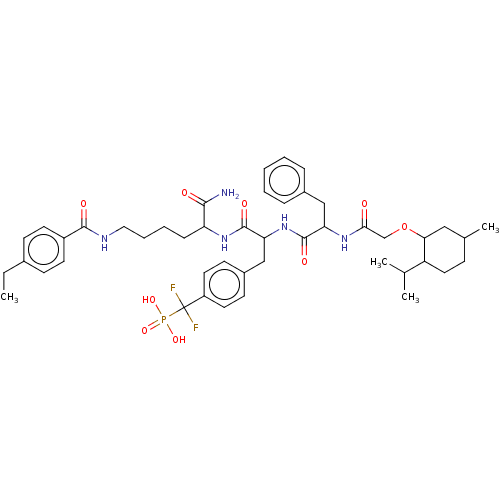
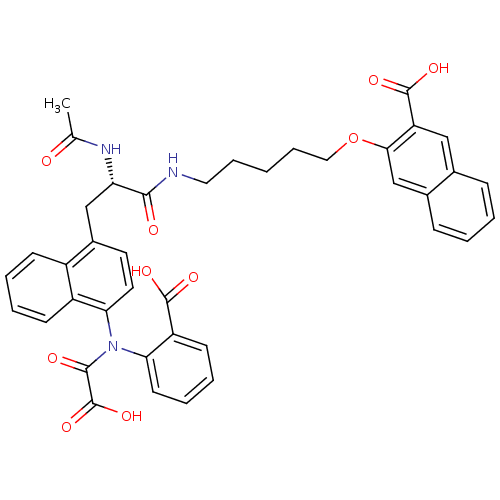
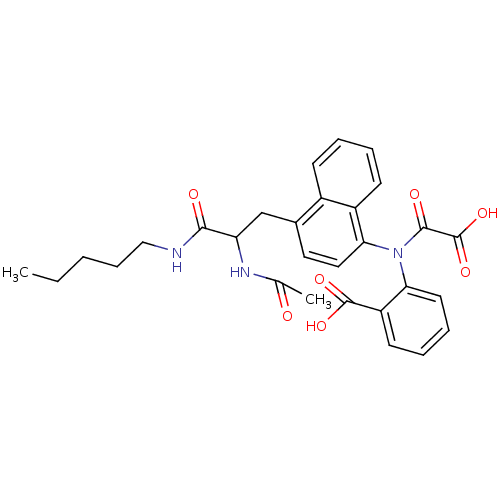
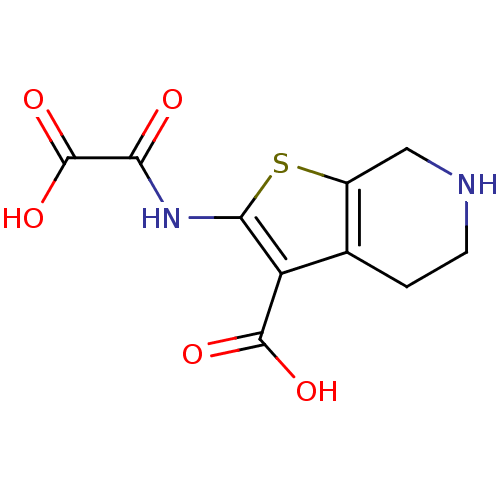
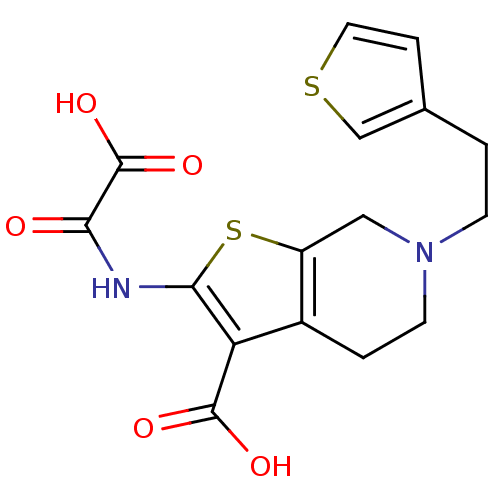
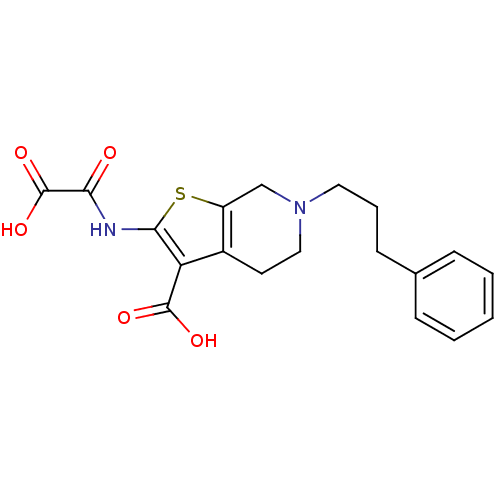
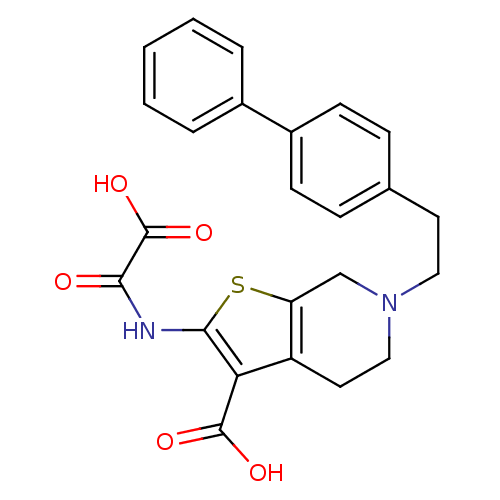
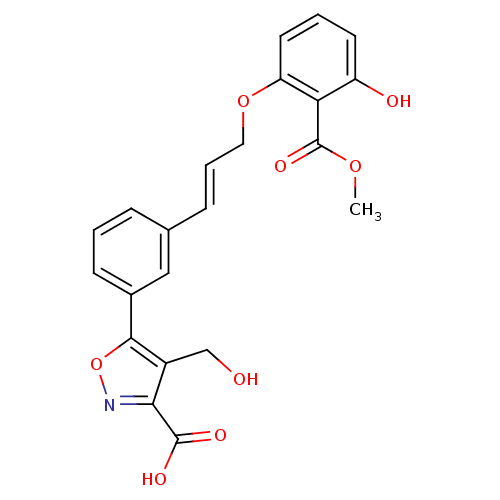
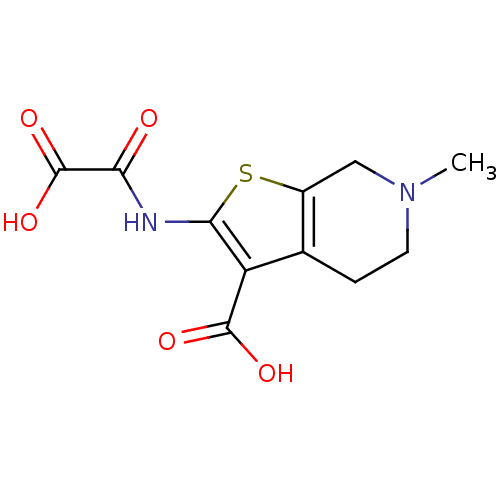
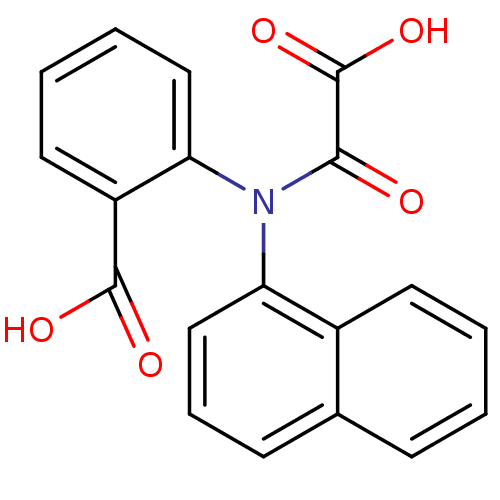
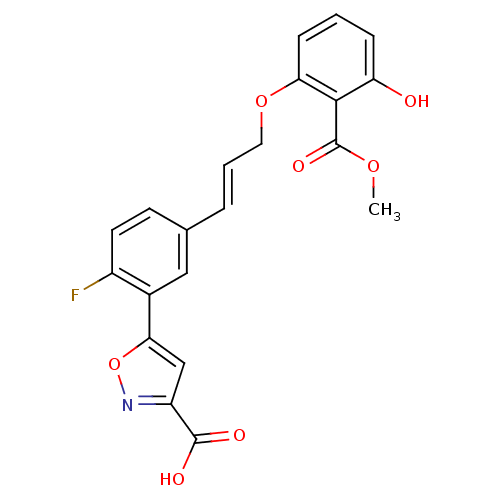
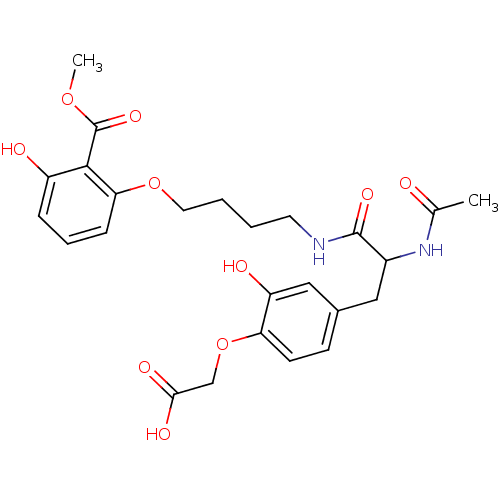
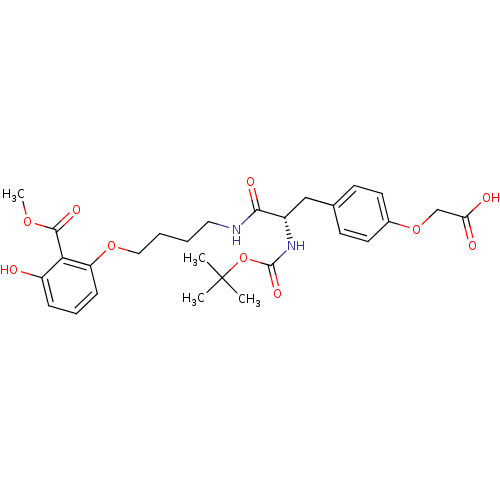
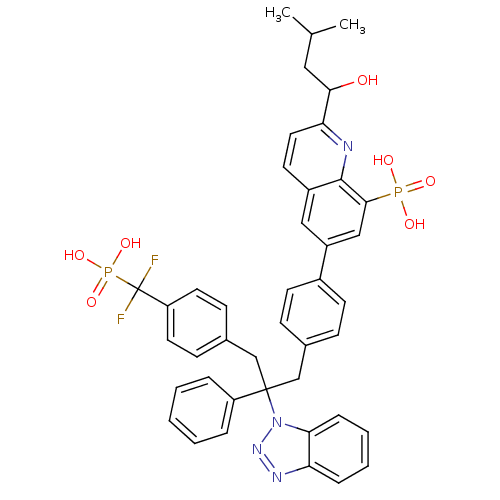
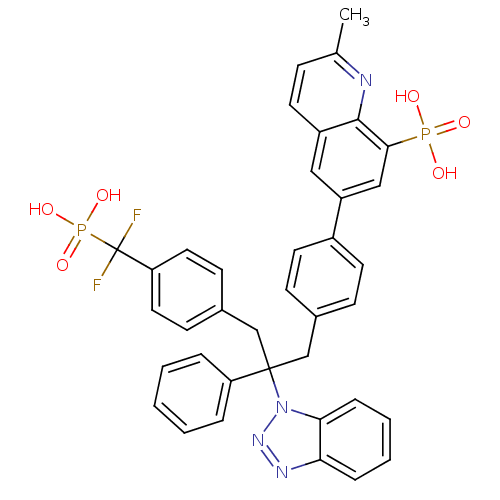
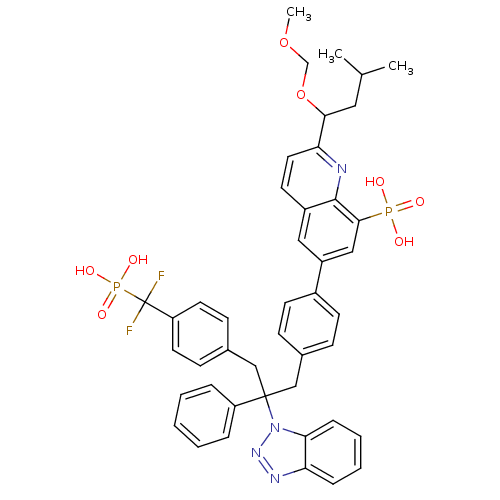
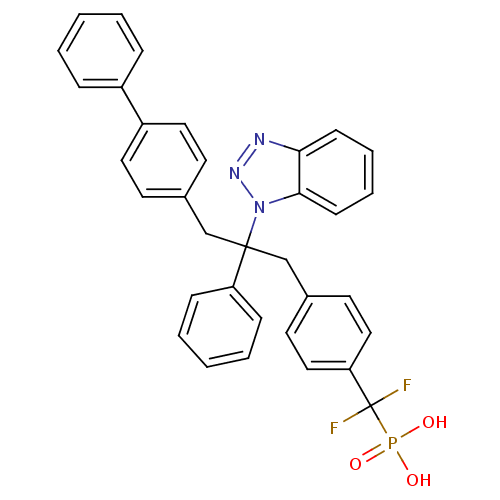
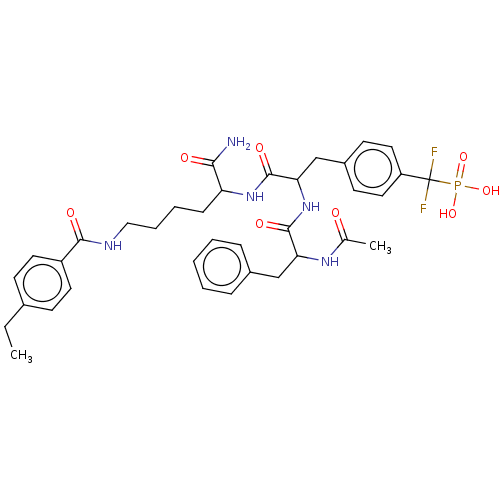
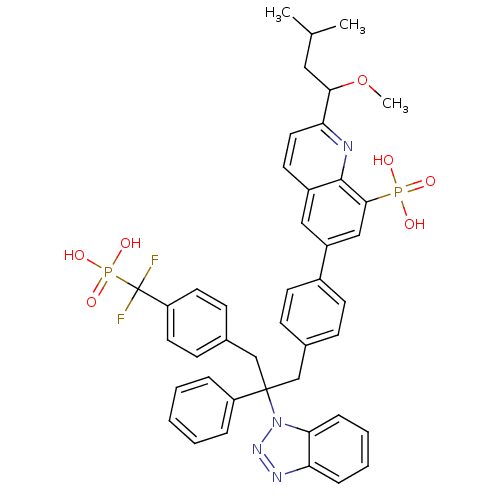
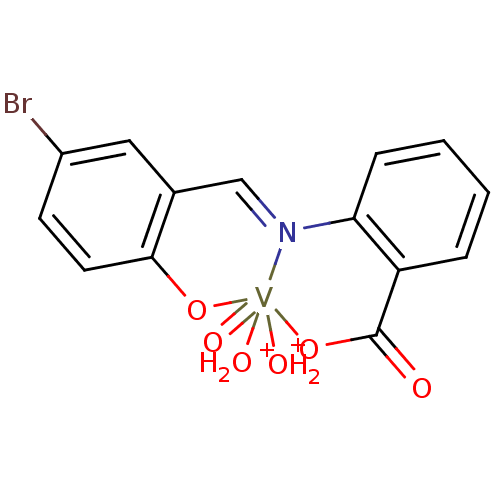
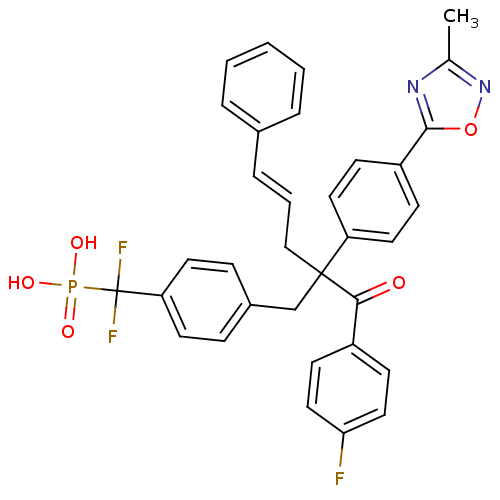
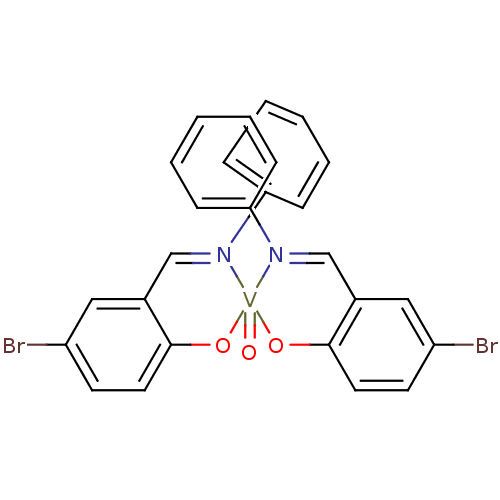
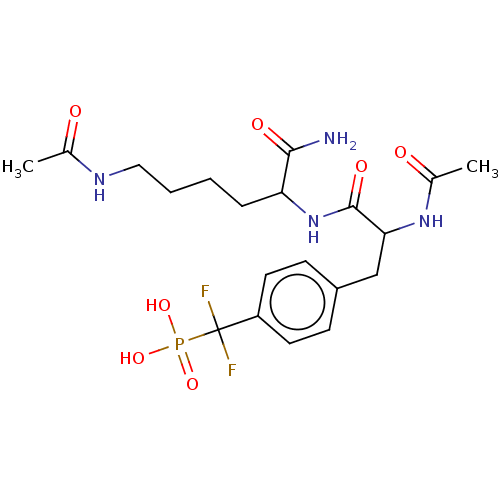
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: 4.10nM ΔG°: -47.9kJ/molepH: 7.0 T: 2°CAssay Description:PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ...More data for this Ligand-Target Pair
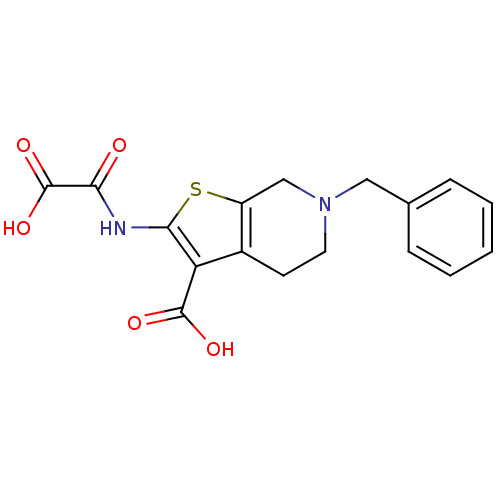
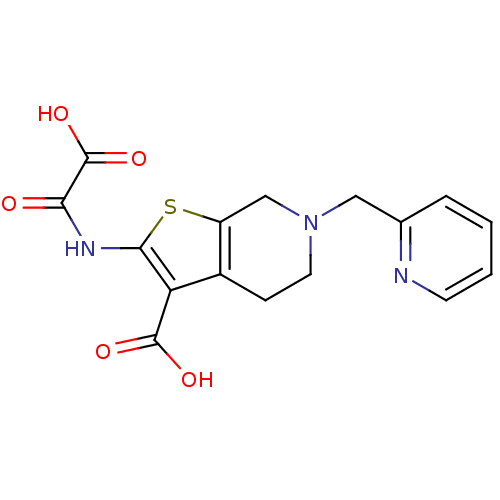
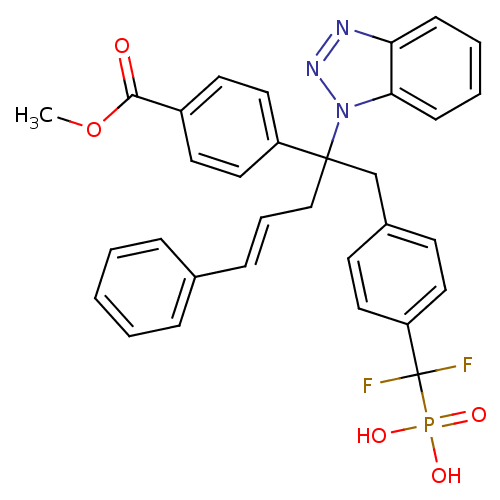
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: 4.30nM ΔG°: -47.8kJ/molepH: 7.0 T: 2°CAssay Description:PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ...More data for this Ligand-Target Pair
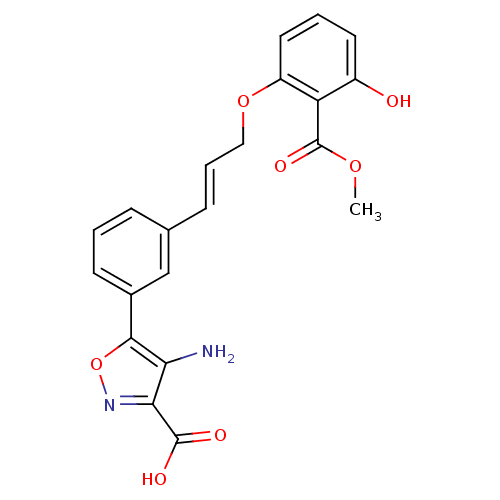
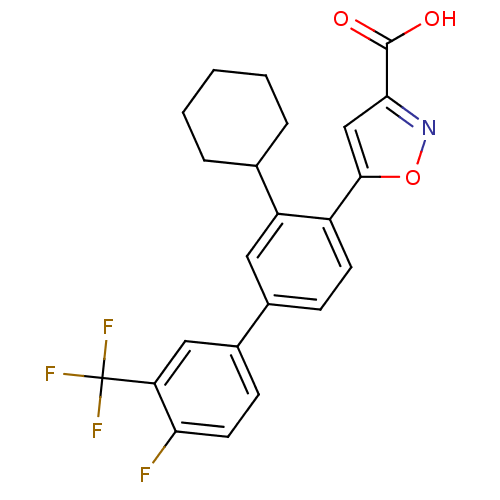
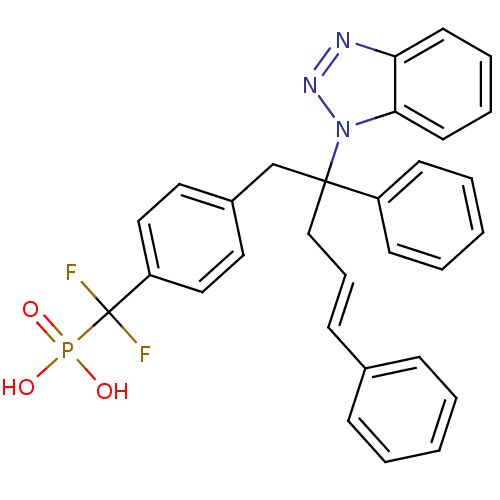
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: 49nM ΔG°: -41.3kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
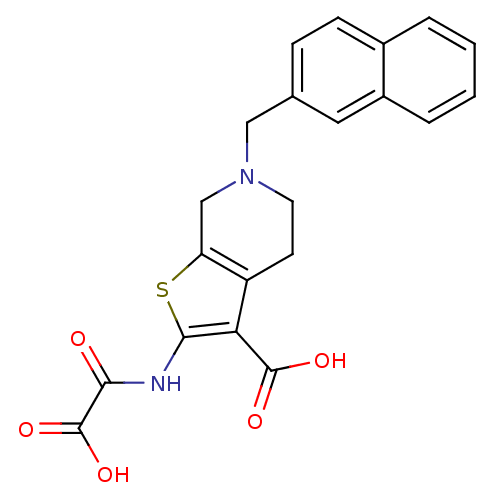
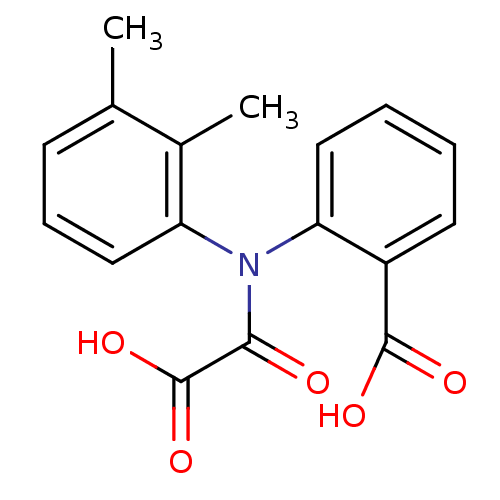
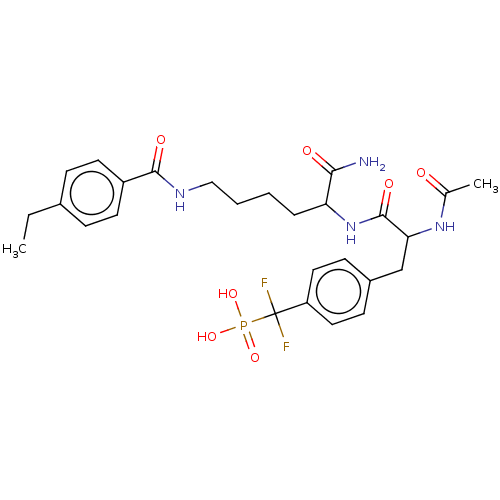
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: 1.10E+3nM ΔG°: -33.7kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: 7.20E+3nMpH: 7.0Assay Description:Inhibition of recombinant TCPTP (unknown origin) at pH 7More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: 9.40E+3nMpH: 7.0Assay Description:Inhibition of T cell protein tyrosine phosphatase (TC-PTP) at pH 7.0More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: 1.00E+4nMpH: 7.0Assay Description:Inhibition of T cell protein tyrosine phosphatase (TC-PTP) at pH 7.0More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: 1.10E+4nMpH: 7.0Assay Description:Inhibition of T cell protein tyrosine phosphatase (TC-PTP) at pH 7.0More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: 1.20E+4nMpH: 7.0Assay Description:Inhibition of T cell protein tyrosine phosphatase (TC-PTP) at pH 7.0More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: 1.20E+4nMpH: 7.0Assay Description:Inhibition of T cell protein tyrosine phosphatase (TC-PTP) at pH 7.0More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: 1.20E+4nMpH: 7.0Assay Description:Inhibition of T cell protein tyrosine phosphatase (TC-PTP) at pH 7.0More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: 1.30E+4nMpH: 7.0Assay Description:Inhibition of T cell protein tyrosine phosphatase (TC-PTP) at pH 7.0More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: 1.70E+4nMpH: 7.0Assay Description:Inhibition of T cell protein tyrosine phosphatase (TC-PTP) at pH 7.0More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: 1.90E+4nMpH: 7.0Assay Description:Inhibition of T cell protein tyrosine phosphatase (TC-PTP) at pH 7.0More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: 1.92E+4nM ΔG°: -26.7kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: 2.20E+4nMpH: 7.0Assay Description:Inhibition of T cell protein tyrosine phosphatase (TC-PTP) at pH 7.0More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: 2.40E+4nMpH: 7.0Assay Description:Inhibition of T cell protein tyrosine phosphatase (TC-PTP) at pH 7.0More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: >3.00E+4nM ΔG°: >-25.6kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: 3.40E+4nMpH: 7.0Assay Description:Inhibitory effect against inhibitory effect against T cell protein tyrosine phosphatase (TC-PTP) using p-nitrophenyl phosphate as substrate at pH 7.0More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: 4.40E+4nM ΔG°: -24.6kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: 4.80E+4nMpH: 7.0Assay Description:Inhibition of T cell protein tyrosine phosphatase (TC-PTP) at pH 7.0More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: >5.00E+4nM ΔG°: >-24.3kJ/molepH: 7.4 T: 2°CAssay Description:The reaction was started by addition of pNPP substrate, and reaction progress was monitored at 405 nm. The initial rate data collected was used for d...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: 6.50E+4nM ΔG°: -23.7kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: 1.41E+5nM ΔG°: -21.8kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: 1.64E+5nM ΔG°: -21.4kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: 1.64E+5nM ΔG°: -21.4kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: 1.82E+5nM ΔG°: -21.1kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataKi: >2.00E+5nM ΔG°: >-20.9kJ/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataIC50: 3nMpH: 6.3 T: 2°CAssay Description:Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataIC50: 11nMpH: 6.3 T: 2°CAssay Description:Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataIC50: 17nMpH: 6.3 T: 2°CAssay Description:Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataIC50: 20nMpH: 6.3 T: 2°CAssay Description:Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataIC50: 20nMpH: 6.3 T: 2°CAssay Description:Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataIC50: 21nMpH: 6.3 T: 2°CAssay Description:Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataIC50: 24nMpH: 6.3 T: 2°CAssay Description:Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataIC50: 26nMpH: 7.0 T: 2°CAssay Description:PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataIC50: 31nMpH: 6.3 T: 2°CAssay Description:Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataIC50: 36nMpH: 6.3 T: 2°CAssay Description:Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataIC50: 87nMpH: 6.3 T: 2°CAssay Description:Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataIC50: 95nMpH: 6.3 T: 2°CAssay Description:Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataIC50: 160nMpH: 7.0 T: 2°CAssay Description:PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataIC50: 190nMpH: 7.2Assay Description:The assays were performed in 20 mM MOPS buffer, pH 7.2, containing 50 mM NaCl and 2 mM GSH. Compounds were dissolved in DMSO and diluted to various c...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataIC50: 830nMpH: 7.0Assay Description:PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in 3,3-dimethylglutarate buffer (50 mM 3,3-dimethylglutarate, pH 7.0, 1 ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataIC50: 1.30E+3nMpH: 7.2Assay Description:The assays were performed in 20 mM MOPS buffer, pH 7.2, containing 50 mM NaCl and 2 mM GSH. Compounds were dissolved in DMSO and diluted to various c...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataIC50: 1.40E+3nMpH: 7.2Assay Description:The assays were performed in 20 mM MOPS buffer, pH 7.2, containing 50 mM NaCl and 2 mM GSH. Compounds were dissolved in DMSO and diluted to various c...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataIC50: 1.60E+3nMpH: 6.3 T: 2°CAssay Description:Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataIC50: 1.90E+3nMpH: 7.2Assay Description:The assays were performed in 20 mM MOPS buffer, pH 7.2, containing 50 mM NaCl and 2 mM GSH. Compounds were dissolved in DMSO and diluted to various c...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataIC50: 2.00E+3nMpH: 7.0 T: 2°CAssay Description:PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataIC50: 2.90E+3nMpH: 6.5Assay Description:Enzymatic activity of enzyme was evaluated using pNPP assay. Briefly, a reaction mixture (100 ÁL) containing 50 mM MOPS (pH 6.5), 2 mM pNPP and recom...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Indiana University Research And Technology
US Patent
Indiana University Research And Technology
US Patent
Affinity DataIC50: 5.30E+3nMpH: 7.0 T: 2°CAssay Description:PTP assay were conducted at 25 C in 96-well plates. Reaction rates were determined using a Variokan plate reader from Thermo Electron. For assay pH...More data for this Ligand-Target Pair