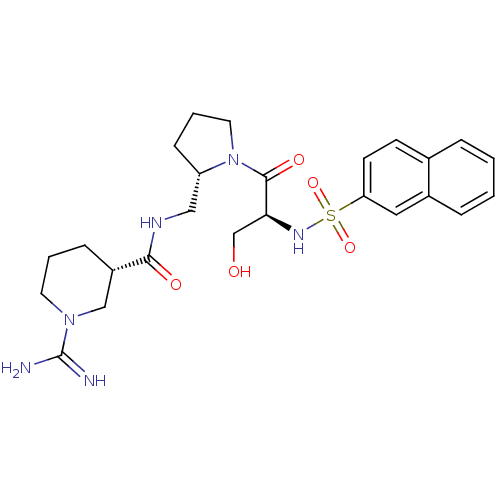
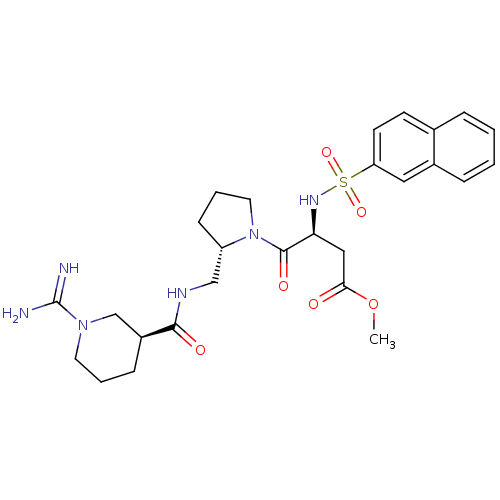
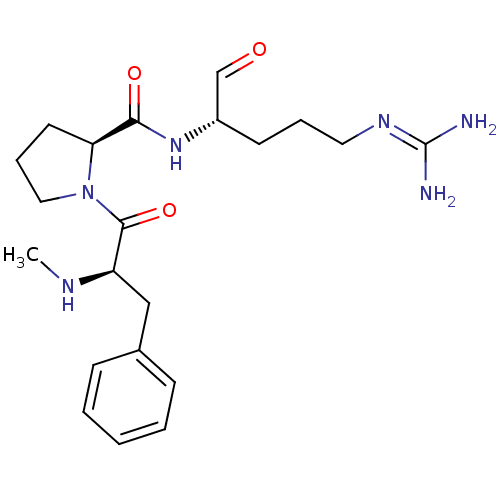
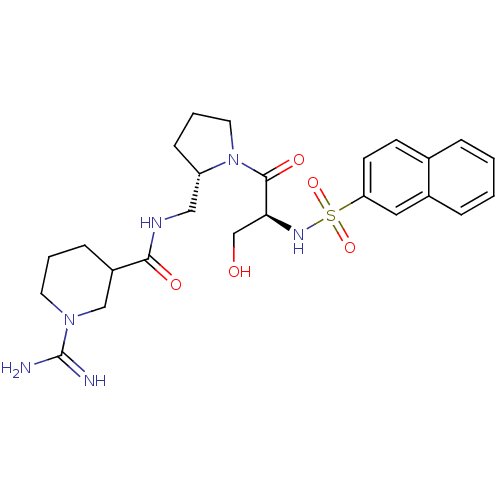
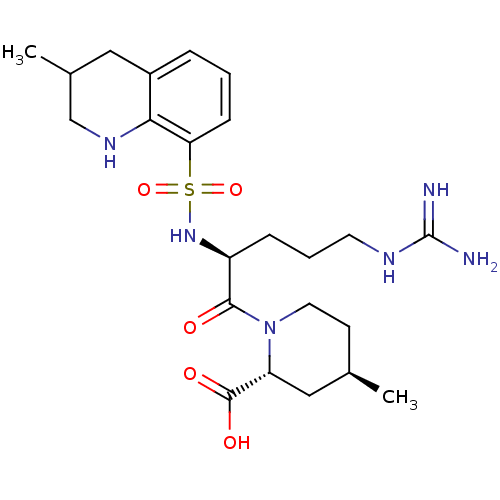
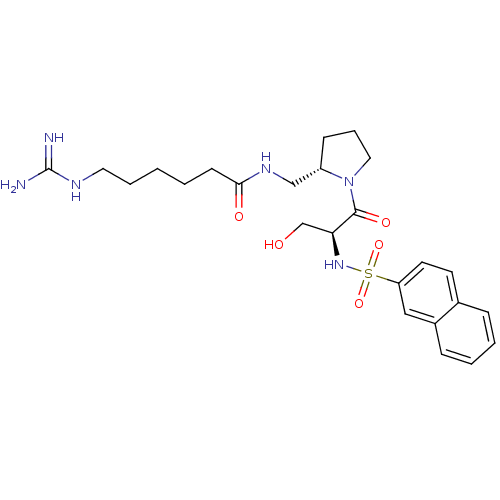
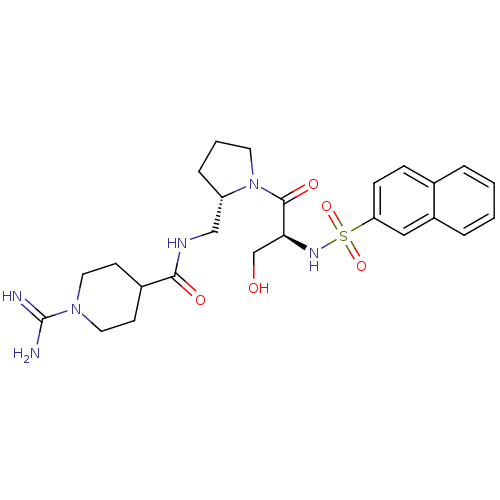
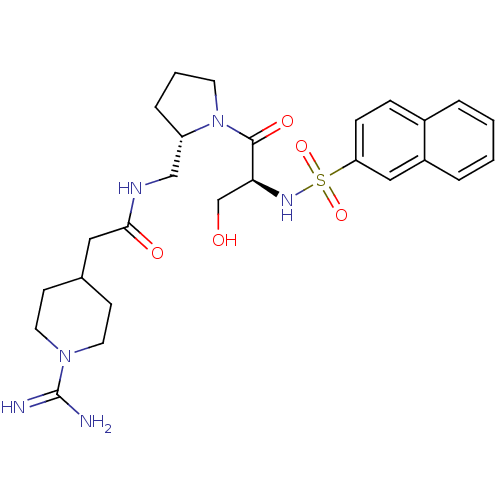
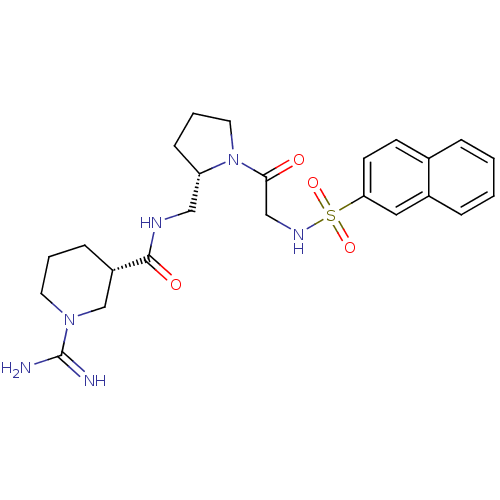
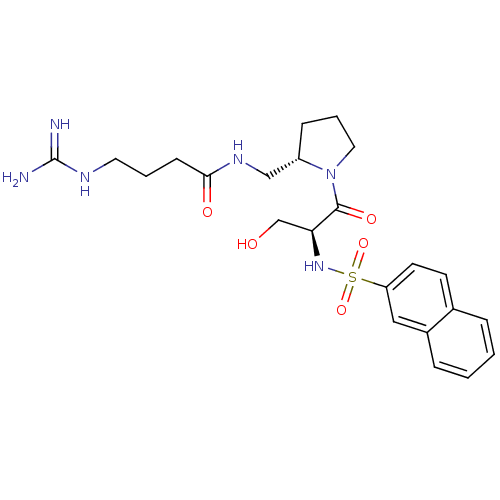
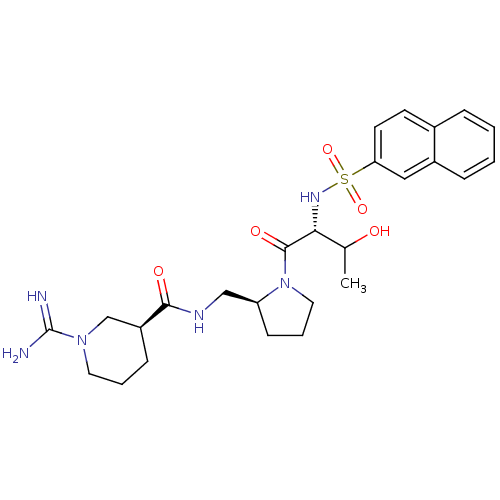
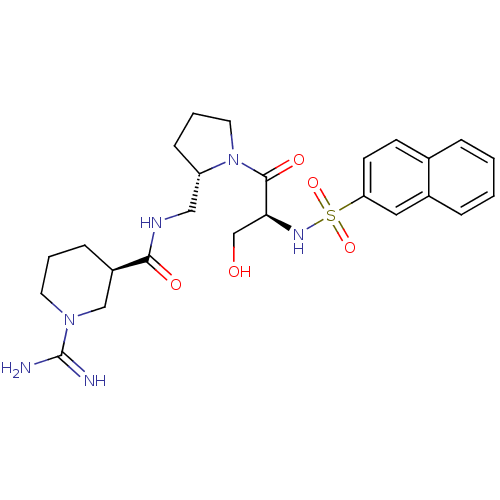
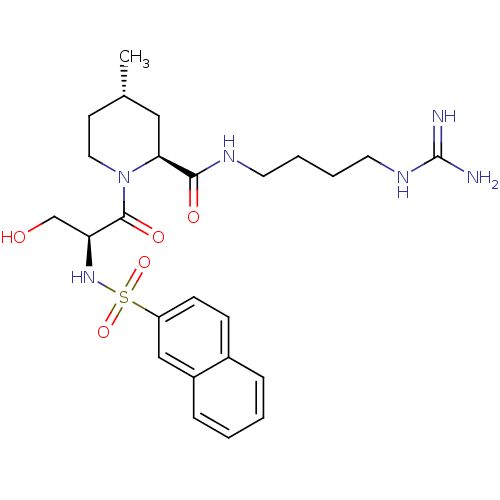
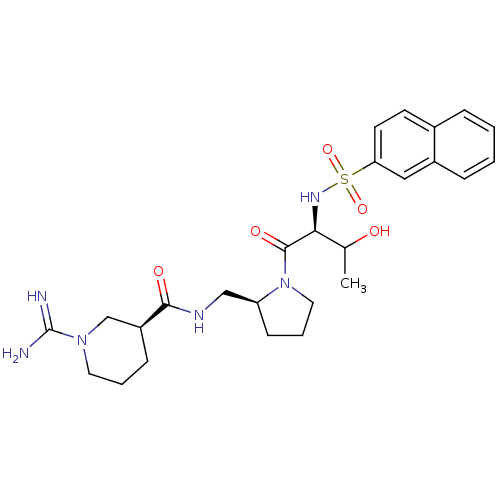
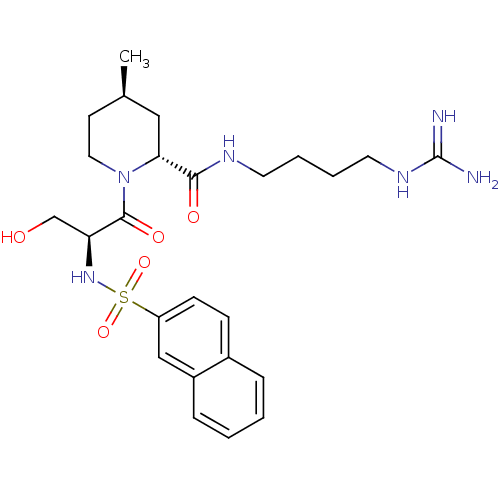
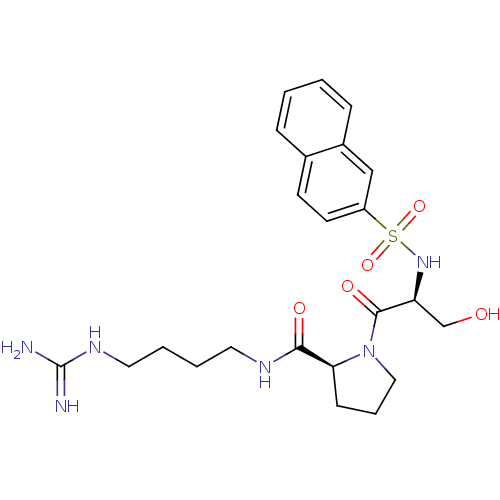
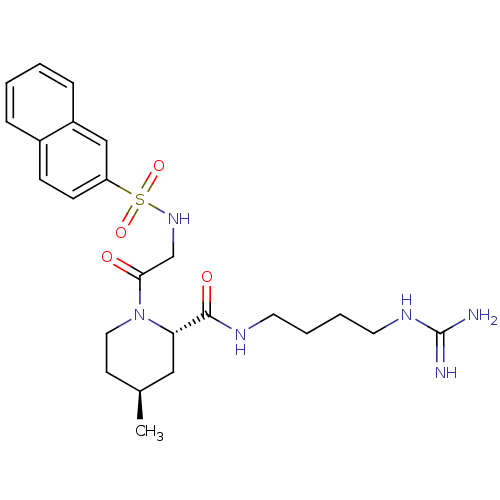
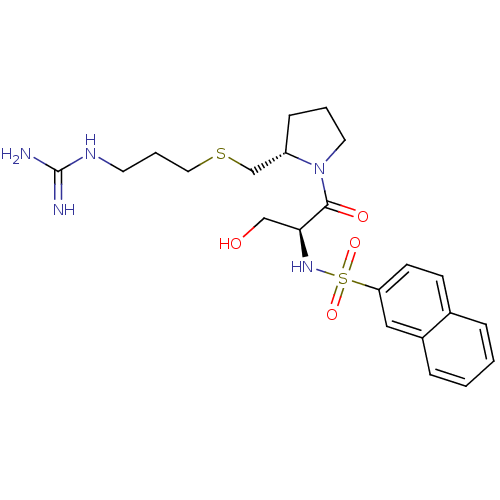
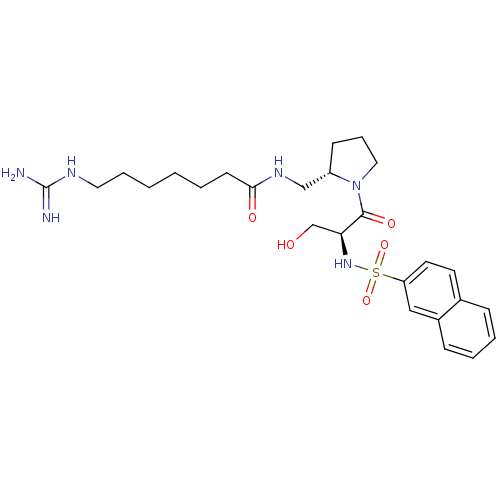
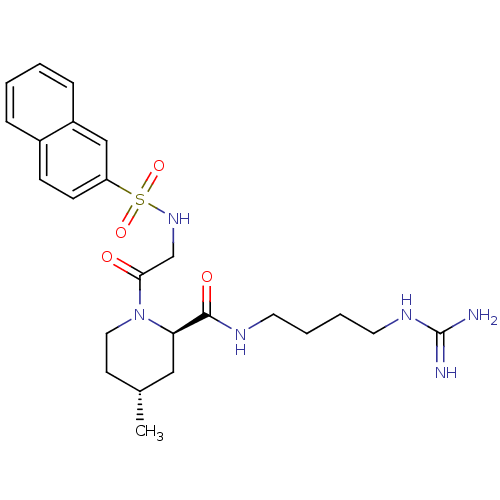
Report error Found 26 Enz. Inhib. hit(s) with all data for entry = 50035218
Affinity DataKi: 3.40nMAssay Description:In vitro reversible inhibition of thrombin catalytic activityMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rt after 3 min incubation with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rt after 3 min incubation with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation.More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:In vitro inhibition of human thrombin catalytic activity after 3 min pre incubation.More data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rt after 3 min incubation with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rt after 3 min incubation with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 38nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rat after 3 min incubation with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rt after 3 min incubation with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 75nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rt after 3 min incubation with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rt after 3 min incubation with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 110nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rt after 3 min incubation with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rt after 3 min incubation with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 310nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rt after 3 min incubation with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 390nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rt after 3 min incubation with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rat after 3 min incubation with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rt after 3 min incubation with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 620nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rt after 3 min incubation with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 650nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rt after 3 min incubation with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rt after 3 min incubation with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rt after 3 min incubation with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+3nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rt after 3 min incubation with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 2.90E+3nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rt after 3 min incubation with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 7.40E+3nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rt after 3 min incubation with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+4nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rt after 3 min incubation with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+4nMAssay Description:In vitro inhibition of thrombin catalytic activity using s-2238 substrate at 10 uM was measured at rt after 3 min incubation with compoundMore data for this Ligand-Target Pair