TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The Johns Hopkins University
US Patent
The Johns Hopkins University
US Patent
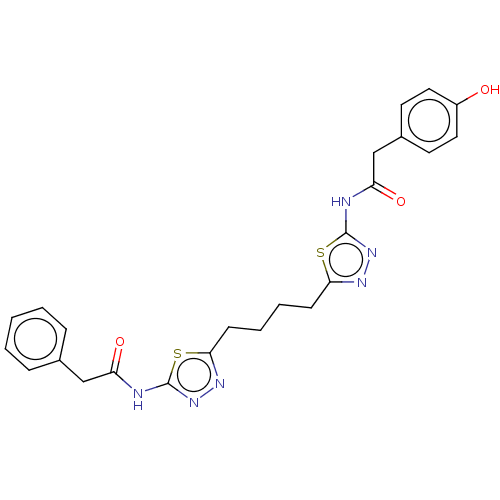
Affinity DataIC50: 80nMAssay Description:A high throughput screening (HTS) assay and together with NCATS screened over 350,000 compounds in attempt to identify novel GLS inhibitor structures...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The Johns Hopkins University
US Patent
The Johns Hopkins University
US Patent
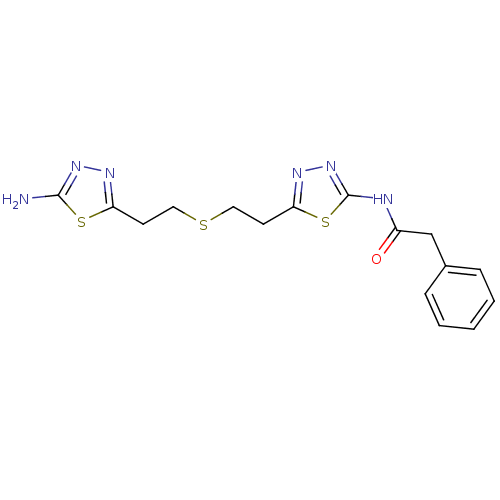
Affinity DataIC50: 100nMAssay Description:A high throughput screening (HTS) assay and together with NCATS screened over 350,000 compounds in attempt to identify novel GLS inhibitor structures...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The Johns Hopkins University
US Patent
The Johns Hopkins University
US Patent
Affinity DataIC50: 100nMAssay Description:A high throughput screening (HTS) assay and together with NCATS screened over 350,000 compounds in attempt to identify novel GLS inhibitor structures...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The Johns Hopkins University
US Patent
The Johns Hopkins University
US Patent
Affinity DataIC50: 1.90E+3nMAssay Description:A high throughput screening (HTS) assay and together with NCATS screened over 350,000 compounds in attempt to identify novel GLS inhibitor structures...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The Johns Hopkins University
US Patent
The Johns Hopkins University
US Patent
Affinity DataIC50: 2.70E+3nMAssay Description:A high throughput screening (HTS) assay and together with NCATS screened over 350,000 compounds in attempt to identify novel GLS inhibitor structures...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The Johns Hopkins University
US Patent
The Johns Hopkins University
US Patent
Affinity DataIC50: 3.30E+3nMAssay Description:A high throughput screening (HTS) assay and together with NCATS screened over 350,000 compounds in attempt to identify novel GLS inhibitor structures...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The Johns Hopkins University
US Patent
The Johns Hopkins University
US Patent
Affinity DataIC50: 3.30E+3nMAssay Description:A high throughput screening (HTS) assay and together with NCATS screened over 350,000 compounds in attempt to identify novel GLS inhibitor structures...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The Johns Hopkins University
US Patent
The Johns Hopkins University
US Patent
Affinity DataIC50: 4.60E+3nMAssay Description:A high throughput screening (HTS) assay and together with NCATS screened over 350,000 compounds in attempt to identify novel GLS inhibitor structures...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The Johns Hopkins University
US Patent
The Johns Hopkins University
US Patent
Affinity DataIC50: 1.00E+5nMAssay Description:A high throughput screening (HTS) assay and together with NCATS screened over 350,000 compounds in attempt to identify novel GLS inhibitor structures...More data for this Ligand-Target Pair





 3D Structure (crystal)
3D Structure (crystal)
