Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 3A4
Ligand
BDBM27879
Substrate
n/a
Meas. Tech.
ChEMBL_321420 (CHEMBL881951)
IC50
500±n/a nM
Citation
 Wittman, M; Carboni, J; Attar, R; Balasubramanian, B; Balimane, P; Brassil, P; Beaulieu, F; Chang, C; Clarke, W; Dell, J; Eummer, J; Frennesson, D; Gottardis, M; Greer, A; Hansel, S; Hurlburt, W; Jacobson, B; Krishnananthan, S; Lee, FY; Li, A; Lin, TA; Liu, P; Ouellet, C; Sang, X; Saulnier, MG; Stoffan, K; Sun, Y; Velaparthi, U; Wong, H; Yang, Z; Zimmermann, K; Zoeckler, M; Vyas, D Discovery of a (1H-benzoimidazol-2-yl)-1H-pyridin-2-one (BMS-536924) inhibitor of insulin-like growth factor I receptor kinase with in vivo antitumor activity. J Med Chem 48:5639-43 (2005) [PubMed] Article
Wittman, M; Carboni, J; Attar, R; Balasubramanian, B; Balimane, P; Brassil, P; Beaulieu, F; Chang, C; Clarke, W; Dell, J; Eummer, J; Frennesson, D; Gottardis, M; Greer, A; Hansel, S; Hurlburt, W; Jacobson, B; Krishnananthan, S; Lee, FY; Li, A; Lin, TA; Liu, P; Ouellet, C; Sang, X; Saulnier, MG; Stoffan, K; Sun, Y; Velaparthi, U; Wong, H; Yang, Z; Zimmermann, K; Zoeckler, M; Vyas, D Discovery of a (1H-benzoimidazol-2-yl)-1H-pyridin-2-one (BMS-536924) inhibitor of insulin-like growth factor I receptor kinase with in vivo antitumor activity. J Med Chem 48:5639-43 (2005) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 3A4
Synonyms:
Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase
Type:
Enzyme
Mol. Mass.:
57349.57
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
503
Sequence:
MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMFDMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISIAEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYSMDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICVFPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSIIFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVVNETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFSKKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLGGLLQPEKPVVLKVESRDGTVSGA
Inhibitor
Name:
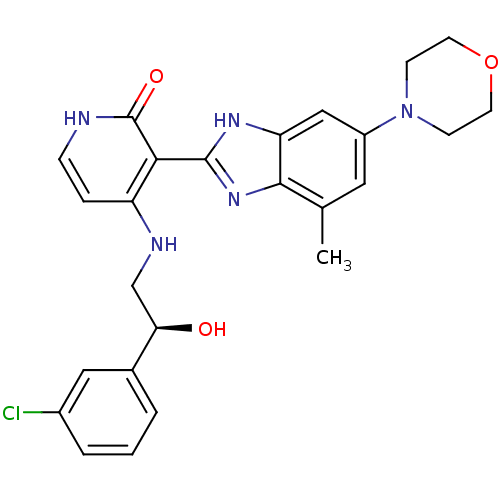
BDBM27879
Synonyms:
4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-3-[4-methyl-6-(morpholin-4-yl)-1H-1,3-benzodiazol-2-yl]-1,2-dihydropyridin-2-one | BMS-536924 | CHEMBL401930 | US10710978, Compound BMS536924
Type:
Small organic molecule
Emp. Form.:
C25H26ClN5O3
Mol. Mass.:
479.959
SMILES:
Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCOCC1 |r|