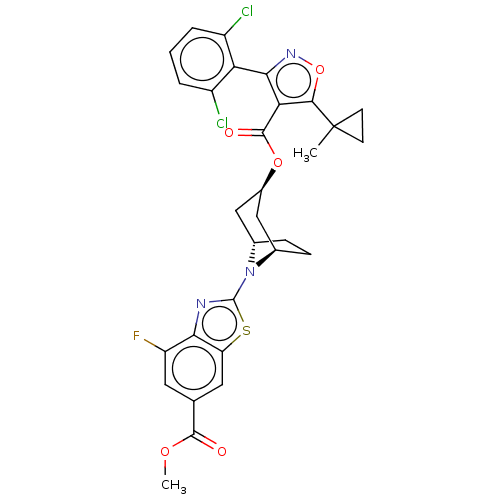
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Bile acid receptor
Ligand
BDBM513471
Substrate
n/a
Meas. Tech.
Ligand Binding Assay
EC50
39.8±n/a nM
Citation
 Chao, J; Jain, R; Hu, L; Lewis, JG; Baribault, H; Caldwell, J Isoxazolyl-carbonyloxy azabicyclo[3.2.1]octanyl compounds as FXR activators US Patent US11091482 Publication Date 8/17/2021
Chao, J; Jain, R; Hu, L; Lewis, JG; Baribault, H; Caldwell, J Isoxazolyl-carbonyloxy azabicyclo[3.2.1]octanyl compounds as FXR activators US Patent US11091482 Publication Date 8/17/2021 More Info.:
Target
Name:
Bile acid receptor
Synonyms:
BAR | Bile acid receptor FXR | FXR | Farnesol receptor HRR-1 | HRR1 | NR1H4 | NR1H4_HUMAN | Nuclear receptor subfamily 1 group H member 4 | RIP14 | RXR-interacting protein 14 | Retinoid X receptor-interacting protein 14 | farnesoid x receptor
Type:
Nuclear Receptor
Mol. Mass.:
55916.24
Organism:
Homo sapiens (Human)
Description:
Q96RI1
Residue:
486
Sequence:
MVMQFQGLENPIQISPHCSCTPSGFFMEMMSMKPAKGVLTEQVAGPLGQNLEVEPYSQYSNVQFPQVQPQISSSSYYSNLGFYPQQPEEWYSPGIYELRRMPAETLYQGETEVAEMPVTKKPRMGASAGRIKGDELCVVCGDRASGYHYNALTCEGCKGFFRRSITKNAVYKCKNGGNCVMDMYMRRKCQECRLRKCKEMGMLAECMYTGLLTEIQCKSKRLRKNVKQHADQTVNEDSEGRDLRQVTSTTKSCREKTELTPDQQTLLHFIMDSYNKQRMPQEITNKILKEEFSAEENFLILTEMATNHVQVLVEFTKKLPGFQTLDHEDQIALLKGSAVEAMFLRSAEIFNKKLPSGHSDLLEERIRNSGISDEYITPMFSFYKSIGELKMTQEEYALLTAIVILSPDRQYIKDREAVEKLQEPLLDVLQKLCKIHQPENPQHFACLLGRLTELRTFNHHHAEMLMSWRVNDHKFTPLLCEIWDVQ
Inhibitor
Name:
BDBM513471
Synonyms:
US11091482, Compound I-26
Type:
Small organic molecule
Emp. Form.:
C30H26Cl2FN3O5S
Mol. Mass.:
630.514
SMILES:
COC(=O)c1cc(F)c2nc(sc2c1)N1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)c1c(noc1C1(C)CC1)-c1c(Cl)cccc1Cl |r,wU:18.20,wD:15.16,20.25,THB:22:20:14:16.17,(.4,15.11,;.4,13.57,;-.94,12.8,;-2.27,13.57,;-.94,11.26,;.4,10.49,;.4,8.95,;1.73,8.18,;-.94,8.18,;-1.26,6.68,;-2.79,6.52,;-3.42,7.92,;-2.27,8.95,;-2.27,10.49,;-3.56,5.18,;-4.76,4.21,;-6.39,4.41,;-5.21,3.75,;-3.77,3.87,;-2.9,2.37,;-4.1,1.4,;-3.87,2.63,;-4.89,.08,;-4.14,-1.27,;-2.6,-1.29,;-4.93,-2.59,;-4.33,-4.01,;-5.49,-5.02,;-6.81,-4.23,;-6.47,-2.73,;-7.48,-1.57,;-6.51,-.37,;-8.83,-.82,;-8.8,-2.36,;-2.83,-4.35,;-1.78,-3.22,;-2.23,-1.75,;-.28,-3.56,;.18,-5.03,;-.87,-6.16,;-2.37,-5.82,;-3.42,-6.95,)|