Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Taste receptor type 1 member 1/3
Ligand
BDBM18137
Substrate
n/a
Meas. Tech.
Activation of T1R1/T1R3 Receptor by Nucleotide Derivatives (alanine)
EC50
11000±n/a nM
Citation
 McGrane, SJ; Taylor, AJ; Fine, RM; Klebansky, B; Gibbs, MR Methods for modulating taste receptors US Patent US11185100 Publication Date 11/30/2021
McGrane, SJ; Taylor, AJ; Fine, RM; Klebansky, B; Gibbs, MR Methods for modulating taste receptors US Patent US11185100 Publication Date 11/30/2021 More Info.:
Target
Name:
Taste receptor type 1 member 1/3
Synonyms:
Taste Receptor T1R1/T1R3
Type:
Enzyme
Mol. Mass.:
n/a
Description:
n/a
Components:
This complex has 2 components.
Component 1
Name:
Taste receptor type 1 member 1
Synonyms:
GPR70 | T1R1 | TAS1R1 | TR1 | TS1R1_HUMAN | Taste receptor type 1 member 1 (T1R1)
Type:
Enzyme
Mol. Mass.:
93094.35
Organism:
Homo sapiens (Human)
Description:
Q7RTX1
Residue:
841
Sequence:
MLLCTARLVGLQLLISCCWAFACHSTESSPDFTLPGDYLLAGLFPLHSGCLQVRHRPEVTLCDRSCSFNEHGYHLFQAMRLGVEEINNSTALLPNITLGYQLYDVCSDSANVYATLRVLSLPGQHHIELQGDLLHYSPTVLAVIGPDSTNRAATTAALLSPFLVPMISYAASSETLSVKRQYPSFLRTIPNDKYQVETMVLLLQKFGWTWISLVGSSDDYGQLGVQALENQATGQGICIAFKDIMPFSAQVGDERMQCLMRHLAQAGATVVVVFSSRQLARVFFESVVLTNLTGKVWVASEAWALSRHITGVPGIQRIGMVLGVAIQKRAVPGLKAFEEAYARADKKAPRPCHKGSWCSSNQLCRECQAFMAHTMPKLKAFSMSSAYNAYRAVYAVAHGLHQLLGCASGACSRGRVYPWQLLEQIHKVHFLLHKDTVAFNDNRDPLSSYNIIAWDWNGPKWTFTVLGSSTWSPVQLNINETKIQWHGKDNQVPKSVCSSDCLEGHQRVVTGFHHCCFECVPCGAGTFLNKSDLYRCQPCGKEEWAPEGSQTCFPRTVVFLALREHTSWVLLAANTLLLLLLLGTAGLFAWHLDTPVVRSAGGRLCFLMLGSLAAGSGSLYGFFGEPTRPACLLRQALFALGFTIFLSCLTVRSFQLIIIFKFSTKVPTFYHAWVQNHGAGLFVMISSAAQLLICLTWLVVWTPLPAREYQRFPHLVMLECTETNSLGFILAFLYNGLLSISAFACSYLGKDLPENYNEAKCVTFSLLFNFVSWIAFFTTASVYDGKYLPAANMMAGLSSLSSGFGGYFLPKCYVILCRPDLNSTEHFQASIQDYTRRCGST
Component 2
Name:
Taste receptor type 1 member 3
Synonyms:
T1R3 | TAS1R3 | TR3 | TS1R3_HUMAN | Taste receptor type 1 member 3 (T1R3)
Type:
Enzyme
Mol. Mass.:
93393.60
Organism:
Homo sapiens (Human)
Description:
Q7RTX0
Residue:
852
Sequence:
MLGPAVLGLSLWALLHPGTGAPLCLSQQLRMKGDYVLGGLFPLGEAEEAGLRSRTRPSSPVCTRFSSNGLLWALAMKMAVEEINNKSDLLPGLRLGYDLFDTCSEPVVAMKPSLMFLAKAGSRDIAAYCNYTQYQPRVLAVIGPHSSELAMVTGKFFSFFLMPQVSYGASMELLSARETFPSFFRTVPSDRVQLTAAAELLQEFGWNWVAALGSDDEYGRQGLSIFSALAAARGICIAHEGLVPLPRADDSRLGKVQDVLHQVNQSSVQVVLLFASVHAAHALFNYSISSRLSPKVWVASEAWLTSDLVMGLPGMAQMGTVLGFLQRGAQLHEFPQYVKTHLALATDPAFCSALGEREQGLEEDVVGQRCPQCDCITLQNVSAGLNHHQTFSVYAAVYSVAQALHNTLQCNASGCPAQDPVKPWQLLENMYNLTFHVGGLPLRFDSSGNVDMEYDLKLWVWQGSVPRLHDVGRFNGSLRTERLKIRWHTSDNQKPVSRCSRQCQEGQVRRVKGFHSCCYDCVDCEAGSYRQNPDDIACTFCGQDEWSPERSTRCFRRRSRFLAWGEPAVLLLLLLLSLALGLVLAALGLFVHHRDSPLVQASGGPLACFGLVCLGLVCLSVLLFPGQPSPARCLAQQPLSHLPLTGCLSTLFLQAAEIFVESELPLSWADRLSGCLRGPWAWLVVLLAMLVEVALCTWYLVAFPPEVVTDWHMLPTEALVHCRTRSWVSFGLAHATNATLAFLCFLGTFLVRSQPGCYNRARGLTFAMLAYFITWVSFVPLLANVQVVLRPAVQMGALLLCVLGILAAFHLPRCYLLMRQPGLNTPEFFLGGGPGDAQGQNDGNTGNQGKHE
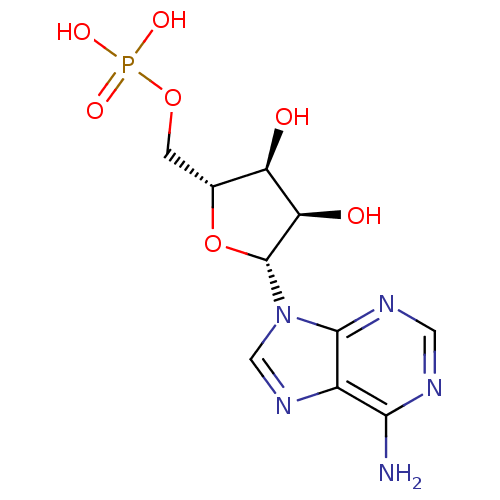
Inhibitor
Name:
BDBM18137
Synonyms:
AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,4R,5R)-5-adenin-9-yl-3,4-dihydroxy-tetrahydrofuran-2-yl]methyl dihydrogen phosphate;hydrate | adenosine 5 -monophosphate | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}phosphonic acid
Type:
Nucleoside or nucleotide
Emp. Form.:
C10H14N5O7P
Mol. Mass.:
347.2212
SMILES:
Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O