Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Voltage-dependent N-type calcium channel subunit alpha-1B
Ligand
BDBM611534
Substrate
n/a
Meas. Tech.
Calcium Channels Interaction
IC50
25.0±n/a nM
Citation
 Villain-Guillot, P; Gualtieri, M; Racine, E Peptide derivatives and uses thereof US Patent US10626144 Publication Date 4/21/2020
Villain-Guillot, P; Gualtieri, M; Racine, E Peptide derivatives and uses thereof US Patent US10626144 Publication Date 4/21/2020 More Info.:
Target
Name:
Voltage-dependent N-type calcium channel subunit alpha-1B
Synonyms:
BIII | Brain calcium channel III | CAC1B_HUMAN | CACH5 | CACNA1B | CACNL1A5 | Calcium channel (Type N) | Calcium channel, L type, alpha-1 polypeptide isoform 5 | Voltage-dependent N-type calcium channel subunit alpha-1B | Voltage-dependent N-type calcium channel subunit alpha-1B/Voltage-dependent calcium channel subunit alpha-2/delta-1/Voltage-dependent L-type calcium channel subunit beta-3 | Voltage-gated N-type calcium channel alpha-1B subunit | Voltage-gated N-type calcium channel alpha-1B subunit/Amyloid beta A4 precursor protein-binding family A member 1 | Voltage-gated calcium channel | Voltage-gated calcium channel subunit alpha Cav2.2 | Voltage-gated calcium channel subunit alpha Cav2.2 ((alpha 1B, beta 1b, alpha 2 delta-1) | calcium channel, voltage-dependent, N type, alpha 1B subunit
Type:
Enzyme
Mol. Mass.:
262548.16
Organism:
Homo sapiens (Human)
Description:
Q00975
Residue:
2339
Sequence:
MVRFGDELGGRYGGPGGGERARGGGAGGAGGPGPGGLQPGQRVLYKQSIAQRARTMALYNPIPVKQNCFTVNRSLFVFSEDNVVRKYAKRITEWPPFEYMILATIIANCIVLALEQHLPDGDKTPMSERLDDTEPYFIGIFCFEAGIKIIALGFVFHKGSYLRNGWNVMDFVVVLTGILATAGTDFDLRTLRAVRVLRPLKLVSGIPSLQVVLKSIMKAMVPLLQIGLLLFFAILMFAIIGLEFYMGKFHKACFPNSTDAEPVGDFPCGKEAPARLCEGDTECREYWPGPNFGITNFDNILFAILTVFQCITMEGWTDILYNTNDAAGNTWNWLYFIPLIIIGSFFMLNLVLGVLSGEFAKERERVENRRAFLKLRRQQQIERELNGYLEWIFKAEEVMLAEEDRNAEEKSPLDVLKRAATKKSRNDLIHAEEGEDRFADLCAVGSPFARASLKSGKTESSSYFRRKEKMFRFFIRRMVKAQSFYWVVLCVVALNTLCVAMVHYNQPRRLTTTLYFAEFVFLGLFLTEMSLKMYGLGPRSYFRSSFNCFDFGVIVGSVFEVVWAAIKPGSSFGISVLRALRLLRIFKVTKYWSSLRNLVVSLLNSMKSIISLLFLLFLFIVVFALLGMQLFGGQFNFQDETPTTNFDTFPAAILTVFQILTGEDWNAVMYHGIESQGGVSKGMFSSFYFIVLTLFGNYTLLNVFLAIAVDNLANAQELTKDEEEMEEAANQKLALQKAKEVAEVSPMSAANISIAARQQNSAKARSVWEQRASQLRLQNLRASCEALYSEMDPEERLRFATTRHLRPDMKTHLDRPLVVELGRDGARGPVGGKARPEAAEAPEGVDPPRRHHRHRDKDKTPAAGDQDRAEAPKAESGEPGAREERPRPHRSHSKEAAGPPEARSERGRGPGPEGGRRHHRRGSPEEAAEREPRRHRAHRHQDPSKECAGAKGERRARHRGGPRAGPREAESGEEPARRHRARHKAQPAHEAVEKETTEKEATEKEAEIVEADKEKELRNHQPREPHCDLETSGTVTVGPMHTLPSTCLQKVEEQPEDADNQRNVTRMGSQPPDPNTIVHIPVMLTGPLGEATVVPSGNVDLESQAEGKKEVEADDVMRSGPRPIVPYSSMFCLSPTNLLRRFCHYIVTMRYFEVVILVVIALSSIALAAEDPVRTDSPRNNALKYLDYIFTGVFTFEMVIKMIDLGLLLHPGAYFRDLWNILDFIVVSGALVAFAFSGSKGKDINTIKSLRVLRVLRPLKTIKRLPKLKAVFDCVVNSLKNVLNILIVYMLFMFIFAVIAVQLFKGKFFYCTDESKELERDCRGQYLDYEKEEVEAQPRQWKKYDFHYDNVLWALLTLFTVSTGEGWPMVLKHSVDATYEEQGPSPGYRMELSIFYVVYFVVFPFFFVNIFVALIIITFQEQGDKVMSECSLEKNERACIDFAISAKPLTRYMPQNRQSFQYKTWTFVVSPPFEYFIMAMIALNTVVLMMKFYDAPYEYELMLKCLNIVFTSMFSMECVLKIIAFGVLNYFRDAWNVFDFVTVLGSITDILVTEIAETNNFINLSFLRLFRAARLIKLLRQGYTIRILLWTFVQSFKALPYVCLLIAMLFFIYAIIGMQVFGNIALDDDTSINRHNNFRTFLQALMLLFRSATGEAWHEIMLSCLSNQACDEQANATECGSDFAYFYFVSFIFLCSFLMLNLFVAVIMDNFEYLTRDSSILGPHHLDEFIRVWAEYDPAACGRISYNDMFEMLKHMSPPLGLGKKCPARVAYKRLVRMNMPISNEDMTVHFTSTLMALIRTALEIKLAPAGTKQHQCDAELRKEISVVWANLPQKTLDLLVPPHKPDEMTVGKVYAALMIFDFYKQNKTTRDQMQQAPGGLSQMGPVSLFHPLKATLEQTQPAVLRGARVFLRQKSSTSLSNGGAIQNQESGIKESVSWGTQRTQDAPHEARPPLERGHSTEIPVGRSGALAVDVQMQSITRRGPDGEPQPGLESQGRAASMPRLAAETQPVTDASPMKRSISTLAQRPRGTHLCSTTPDRPPPSQASSHHHHHRCHRRRDRKQRSLEKGPSLSADMDGAPSSAVGPGLPPGEGPTGCRRERERRQERGRSQERRQPSSSSSEKQRFYSCDRFGGREPPKPKPSLSSHPTSPTAGQEPGPHPQGSGSVNGSPLLSTSGASTPGRGGRRQLPQTPLTPRPSITYKTANSSPIHFAGAQTSLPAFSPGRLSRGLSEHNALLQRDPLSQPLAPGSRIGSDPYLGQRLDSEASVHALPEDTLTFEEAVATNSGRSSRTSYVSSLTSQSHPLRRVPNGYHCTLGLSSGGRARHSYHHPDQDHWC
Inhibitor
Name:
BDBM611534
Synonyms:
US10626144, Compound Odilomycin A
Type:
Small organic molecule
Emp. Form.:
C54H101N23O14
Mol. Mass.:
1296.5254
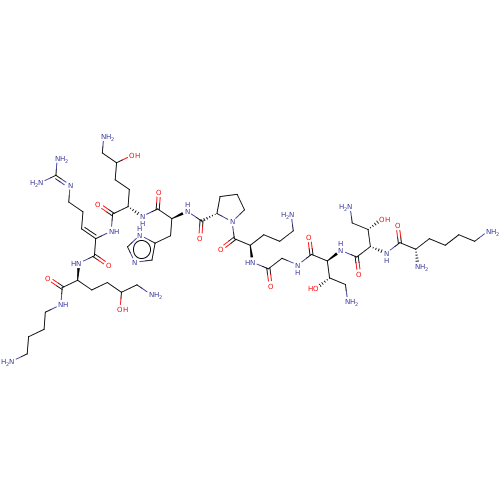
SMILES:
NCCCCNC(=O)[C@H](CCC(O)CN)NC(=O)C(\NC(=O)[C@H](CCC(O)CN)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](CCCN)NC(=O)CNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CCCCN)[C@@H](O)CN)[C@@H](O)CN)=C\CCN=C(N)N |wU:61.63,42.42,22.28,wD:49.55,80.82,76.78,65.67,69.71,32.39,8.14,(-15.75,2.94,;-14.51,3.85,;-13.1,3.22,;-11.86,4.13,;-10.45,3.51,;-9.21,4.42,;-7.8,3.8,;-7.63,2.27,;-6.55,4.71,;-6.72,6.24,;-8.13,6.86,;-8.29,8.39,;-7.05,9.3,;-9.7,9.01,;-9.87,10.55,;-5.14,4.09,;-3.9,5,;-4.07,6.53,;-2.49,4.37,;-2.33,2.84,;-.92,2.22,;.32,3.13,;-.75,.69,;-2,-.22,;-1.83,-1.75,;-3.07,-2.66,;-4.48,-2.04,;-2.91,-4.19,;-4.15,-5.1,;.66,.07,;.82,-1.46,;-.42,-2.37,;2.23,-2.09,;3.47,-1.18,;3.31,.36,;1.97,1.12,;2.29,2.63,;3.82,2.79,;4.45,1.39,;2.4,-3.62,;3.81,-4.24,;5.05,-3.33,;3.97,-5.77,;2.83,-6.8,;3.46,-8.21,;4.99,-8.04,;5.31,-6.53,;6.71,-5.9,;6.87,-4.37,;7.96,-6.81,;7.81,-8.34,;6.4,-8.97,;6.24,-10.5,;4.84,-11.13,;9.37,-6.17,;10.62,-7.08,;10.46,-8.61,;12.02,-6.44,;13.27,-7.35,;14.67,-6.72,;14.83,-5.18,;15.92,-7.62,;17.33,-6.99,;18.58,-7.89,;18.42,-9.42,;19.98,-7.26,;21.23,-8.16,;22.63,-7.53,;22.79,-5.99,;23.88,-8.43,;23.73,-9.96,;25.29,-7.8,;26.54,-8.7,;27.94,-8.07,;29.19,-8.97,;30.6,-8.34,;20.14,-5.72,;21.54,-5.09,;18.89,-4.82,;19.04,-3.29,;15.77,-9.15,;14.36,-9.78,;17.02,-10.05,;16.86,-11.58,;-1.25,5.28,;.16,4.66,;1.4,5.57,;2.81,4.95,;4.05,5.86,;5.46,5.24,;3.89,7.39,)|