Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
Ligand
BDBM50319165
Substrate
n/a
Meas. Tech.
ChEMBL_978080 (CHEMBL2422528)
IC50
32±n/a nM
Citation
 Kilburn, JP; Kehler, J; Langgård, M; Erichsen, MN; Leth-Petersen, S; Larsen, M; Christoffersen, CT; Nielsen, J N-Methylanilide and N-methylbenzamide derivatives as phosphodiesterase 10A (PDE10A) inhibitors. Bioorg Med Chem 21:6053-62 (2013) [PubMed] Article
Kilburn, JP; Kehler, J; Langgård, M; Erichsen, MN; Leth-Petersen, S; Larsen, M; Christoffersen, CT; Nielsen, J N-Methylanilide and N-methylbenzamide derivatives as phosphodiesterase 10A (PDE10A) inhibitors. Bioorg Med Chem 21:6053-62 (2013) [PubMed] Article More Info.:
Target
Name:
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
Synonyms:
3',5'-cyclic phosphodiesterase | 3.1.4.17 | PDE10A | PDE10_HUMAN | Phosphodiesterase 10 (PDE10) | Phosphodiesterase 10A
Type:
Protein
Mol. Mass.:
88412.52
Organism:
Homo sapiens (Human)
Description:
Q9Y233
Residue:
1055
Sequence:
MASLEEPLAPRPQGPLPAAGDEPGCGPGKLRPEPRLSAAGGGSAAGPGPAPEWPGRGRAERAAPPRPPLSSAGRPSPAGGPGALSARGGGCGWVAARAPLALAFSSRVPSSSPSFFYFWPPPPPPPPSFLPSSSAFHLPVRLPGREGAAAAAAAGGGGDAGGGGGGGQEAAPLSVPTSSSHRGGGGSGGGRRRLFLSPALQGLLLPARAGPRPPPPPRLPLGQAARRAGSPGFPGAGPGGGGQTPRRPQGASFALAAAAALLFGSDMEDGPSNNASCFRRLTECFLSPSLTDEKVKAYLSLHPQVLDEFVSESVSAETVEKWLKRKNNKSEDESAPKEVSRYQDTNMQGVVYELNSYIEQRLDTGGDNQLLLYELSSIIKIATKADGFALYFLGECNNSLCIFTPPGIKEGKPRLIPAGPITQGTTVSAYVAKSRKTLLVEDILGDERFPRGTGLESGTRIQSVLCLPIVTAIGDLIGILELYRHWGKEAFCLSHQEVATANLAWASVAIHQVQVCRGLAKQTELNDFLLDVSKTYFDNIVAIDSLLEHIMIYAKNLVNADRCALFQVDHKNKELYSDLFDIGEEKEGKPVFKKTKEIRFSIEKGIAGQVARTGEVLNIPDAYADPRFNREVDLYTGYTTRNILCMPIVSRGSVIGVVQMVNKISGSAFSKTDENNFKMFAVFCALALHCANMYHRIRHSECIYRVTMEKLSYHSICTSEEWQGLMQFTLPVRLCKEIELFHFDIGPFENMWPGIFVYMVHRSCGTSCFELEKLCRFIMSVKKNYRRVPYHNWKHAVTVAHCMYAILQNNHTLFTDLERKGLLIACLCHDLDHRGFSNSYLQKFDHPLAALYSTSTMEQHHFSQTVSILQLEGHNIFSTLSSSEYEQVLEIIRKAIIATDLALYFGNRKQLEEMYQTGSLNLNNQSHRDRVIGLMMTACDLCSVTKLWPVTKLTANDIYAEFWAEGDEMKKLGIQPIPMMDRDKKDEVPQGQLGFYNAVAIPCYTTLTQILPPTEPLLKACRDNLSQWEKVIRGEETATWISSPSVAQKAAASED
Inhibitor
Name:
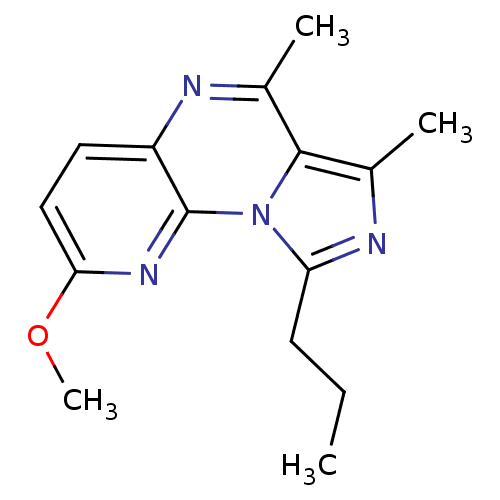
BDBM50319165
Synonyms:
2-methoxy-6,7-dimethyl-9-propylimidazo[1,5-a]pyrido[3,2-e]pyrazine | 3,4-Dimethyl-8-methoxy-1-propyl-imidazo[1,5-a]pyrido[3,2-e]pyrazine | CHEMBL1086110
Type:
Small organic molecule
Emp. Form.:
C15H18N4O
Mol. Mass.:
270.3296
SMILES:
CCCc1nc(C)c2c(C)nc3ccc(OC)nc3n12