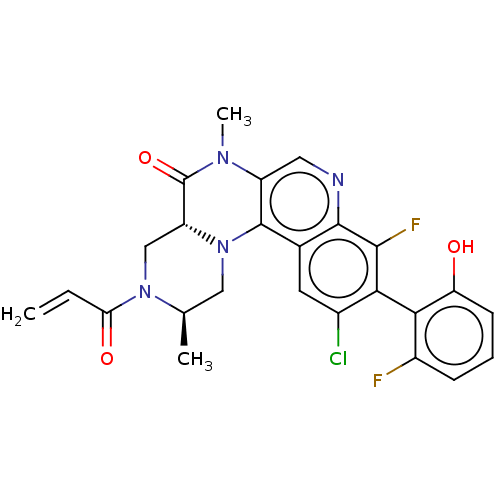
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
GTPase KRas
Ligand
BDBM50527057
Substrate
n/a
Meas. Tech.
ChEMBL_1901970 (CHEMBL4404192)
IC50
<5.0±n/a nM
Citation
 Kettle, JG; Bagal, SK; Bickerton, S; Bodnarchuk, MS; Breed, J; Carbajo, RJ; Cassar, DJ; Chakraborty, A; Cosulich, S; Cumming, I; Davies, M; Eatherton, A; Evans, L; Feron, L; Fillery, S; Gleave, ES; Goldberg, FW; Harlfinger, S; Hanson, L; Howard, M; Howells, R; Jackson, A; Kemmitt, P; Kingston, JK; Lamont, S; Lewis, HJ; Li, S; Liu, L; Ogg, D; Phillips, C; Polanski, R; Robb, G; Robinson, D; Ross, S; Smith, JM; Tonge, M; Whiteley, R; Yang, J; Zhang, L; Zhao, X Structure-Based Design and Pharmacokinetic Optimization of Covalent Allosteric Inhibitors of the Mutant GTPase KRAS J Med Chem 63:4468-4483 (2020) [PubMed] Article
Kettle, JG; Bagal, SK; Bickerton, S; Bodnarchuk, MS; Breed, J; Carbajo, RJ; Cassar, DJ; Chakraborty, A; Cosulich, S; Cumming, I; Davies, M; Eatherton, A; Evans, L; Feron, L; Fillery, S; Gleave, ES; Goldberg, FW; Harlfinger, S; Hanson, L; Howard, M; Howells, R; Jackson, A; Kemmitt, P; Kingston, JK; Lamont, S; Lewis, HJ; Li, S; Liu, L; Ogg, D; Phillips, C; Polanski, R; Robb, G; Robinson, D; Ross, S; Smith, JM; Tonge, M; Whiteley, R; Yang, J; Zhang, L; Zhao, X Structure-Based Design and Pharmacokinetic Optimization of Covalent Allosteric Inhibitors of the Mutant GTPase KRAS J Med Chem 63:4468-4483 (2020) [PubMed] Article More Info.:
Target
Name:
GTPase KRas
Synonyms:
GTPase KRas, N-terminally processed | K-Ras 2 | KRAS | KRAS2 | Ki-Ras | RASK2 | RASK_HUMAN | c-K-ras | c-Ki-ras
Type:
PROTEIN
Mol. Mass.:
21656.10
Organism:
Homo sapiens (Human)
Description:
ChEMBL_1476955
Residue:
189
Sequence:
MTEYKLVVVGAGGVGKSALTIQLIQNHFVDEYDPTIEDSYRKQVVIDGETCLLDILDTAGQEEYSAMRDQYMRTGEGFLCVFAINNTKSFEDIHHYREQIKRVKDSEDVPMVLVGNKCDLPSRTVDTKQAQDLARSYGIPFIETSAKTRQRVEDAFYTLVREIRQYRLKKISKEEKTPGCVKIKKCIIM
Inhibitor
Name:
BDBM50527057
Synonyms:
CHEMBL4461434
Type:
Small organic molecule
Emp. Form.:
C25H21ClF2N4O3
Mol. Mass.:
498.909
SMILES:
[H][C@]12CN([C@H](C)CN1c1c(cnc3c(F)c(c(Cl)cc13)-c1c(O)cccc1F)N(C)C2=O)C(=O)C=C |r,wU:1.0,4.4,(50.79,-26.84,;49.46,-27.62,;49.45,-26.08,;48.12,-25.32,;46.79,-26.1,;45.45,-25.34,;46.8,-27.64,;48.13,-28.4,;48.14,-29.94,;49.48,-30.7,;49.49,-32.25,;48.15,-33.03,;46.82,-32.26,;45.48,-33.04,;45.49,-34.58,;44.15,-32.27,;44.15,-30.72,;42.82,-29.95,;45.48,-29.95,;46.81,-30.71,;42.82,-33.04,;41.49,-32.26,;41.49,-30.72,;40.15,-33.03,;40.15,-34.57,;41.49,-35.34,;42.82,-34.57,;44.15,-35.34,;50.81,-29.92,;52.14,-30.69,;50.8,-28.38,;52.13,-27.61,;48.11,-23.78,;46.77,-23.02,;49.44,-23,;49.43,-21.46,)|