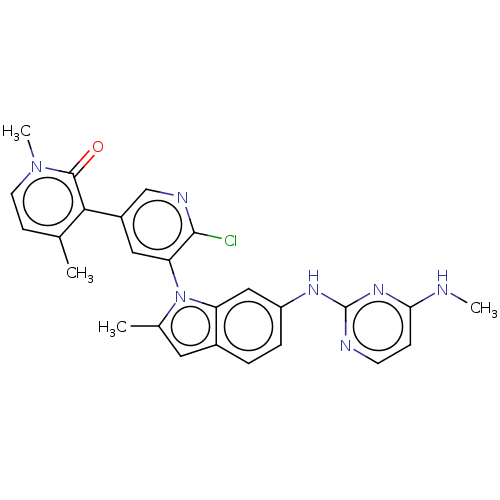
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Histone-lysine N-methyltransferase, H3 lysine-79 specific
Ligand
BDBM50536831
Substrate
n/a
Meas. Tech.
ChEMBL_1934645 (CHEMBL4480297)
IC50
10.0±n/a nM
Citation
 Chen, C; Zhu, H; Stauffer, F; Caravatti, G; Vollmer, S; Machauer, R; Holzer, P; M÷bitz, H; Scheufler, C; Klumpp, M; Tiedt, R; Beyer, KS; Calkins, K; Guthy, D; Kiffe, M; Zhang, J; Gaul, C Discovery of Novel Dot1L Inhibitors through a Structure-Based Fragmentation Approach. ACS Med Chem Lett 7:735-40 (2016) [PubMed] Article
Chen, C; Zhu, H; Stauffer, F; Caravatti, G; Vollmer, S; Machauer, R; Holzer, P; M÷bitz, H; Scheufler, C; Klumpp, M; Tiedt, R; Beyer, KS; Calkins, K; Guthy, D; Kiffe, M; Zhang, J; Gaul, C Discovery of Novel Dot1L Inhibitors through a Structure-Based Fragmentation Approach. ACS Med Chem Lett 7:735-40 (2016) [PubMed] Article More Info.:
Target
Name:
Histone-lysine N-methyltransferase, H3 lysine-79 specific
Synonyms:
2.1.1.43 | DOT1-like protein | DOT1-like protein (Dot1L) | DOT1L | DOT1L_HUMAN | H3-K79-HMTase | Histone H3-K79 methyltransferase | Histone H3-K79 methyltransferase (DOT1L) | Histone Methyltransferase DOT1L | Histone-lysine N-methyltransferase, H3 lysine-79 specific (DOT1L) | KIAA1814 | KMT4 | Lysine N-methyltransferase 4
Type:
Protein
Mol. Mass.:
184911.91
Organism:
Homo sapiens (Human)
Description:
Q8TEK3
Residue:
1537
Sequence:
MGEKLELRLKSPVGAEPAVYPWPLPVYDKHHDAAHEIIETIRWVCEEIPDLKLAMENYVLIDYDTKSFESMQRLCDKYNRAIDSIHQLWKGTTQPMKLNTRPSTGLLRHILQQVYNHSVTDPEKLNNYEPFSPEVYGETSFDLVAQMIDEIKMTDDDLFVDLGSGVGQVVLQVAAATNCKHHYGVEKADIPAKYAETMDREFRKWMKWYGKKHAEYTLERGDFLSEEWRERIANTSVIFVNNFAFGPEVDHQLKERFANMKEGGRIVSSKPFAPLNFRINSRNLSDIGTIMRVVELSPLKGSVSWTGKPVSYYLHTIDRTILENYFSSLKNPKLREEQEAARRRQQRESKSNAATPTKGPEGKVAGPADAPMDSGAEEEKAGAATVKKPSPSKARKKKLNKKGRKMAGRKRGRPKKMNTANPERKPKKNQTALDALHAQTVSQTAASSPQDAYRSPHSPFYQLPPSVQRHSPNPLLVAPTPPALQKLLESFKIQYLQFLAYTKTPQYKASLQELLGQEKEKNAQLLGAAQQLLSHCQAQKEEIRRLFQQKLDELGVKALTYNDLIQAQKEISAHNQQLREQSEQLEQDNRALRGQSLQLLKARCEELQLDWATLSLEKLLKEKQALKSQISEKQRHCLELQISIVELEKSQRQQELLQLKSCVPPDDALSLHLRGKGALGRELEPDASRLHLELDCTKFSLPHLSSMSPELSMNGQAAGYELCGVLSRPSSKQNTPQYLASPLDQEVVPCTPSHVGRPRLEKLSGLAAPDYTRLSPAKIVLRRHLSQDHTVPGRPAASELHSRAEHTKENGLPYQSPSVPGSMKLSPQDPRPLSPGALQLAGEKSSEKGLRERAYGSSGELITSLPISIPLSTVQPNKLPVSIPLASVVLPSRAERARSTPSPVLQPRDPSSTLEKQIGANAHGAGSRSLALAPAGFSYAGSVAISGALAGSPASLTPGAEPATLDESSSSGSLFATVGSRSSTPQHPLLLAQPRNSLPASPAHQLSSSPRLGGAAQGPLPEASKGDLPSDSGFSDPESEAKRRIVFTITTGAGSAKQSPSSKHSPLTASARGDCVPSHGQDSRRRGRRKRASAGTPSLSAGVSPKRRALPSVAGLFTQPSGSPLNLNSMVSNINQPLEITAISSPETSLKSSPVPYQDHDQPPVLKKERPLSQTNGAHYSPLTSDEEPGSEDEPSSARIERKIATISLESKSPPKTLENGGGLAGRKPAPAGEPVNSSKWKSTFSPISDIGLAKSADSPLQASSALSQNSLFTFRPALEEPSADAKLAAHPRKGFPGSLSGADGLSPGTNPANGCTFGGGLAADLSLHSFSDGASLPHKGPEAAGLSSPLSFPSQRGKEGSDANPFLSKRQLDGLAGLKGEGSRGKEAGEGGLPLCGPTDKTPLLSGKAAKARDREVDLKNGHNLFISAAAVPPGSLLSGPGLAPAASSAGGAASSAQTHRSFLGPFPPGPQFALGPMSLQANLGSVAGSSVLQSLFSSVPAAAGLVHVSSAATRLTNSHAMGSFSGVAGGTVGGN
Inhibitor
Name:
BDBM50536831
Synonyms:
CHEMBL4549878
Type:
Small organic molecule
Emp. Form.:
C26H24ClN7O
Mol. Mass.:
485.968
SMILES:
CNc1ccnc(Nc2ccc3cc(C)n(-c4cc(cnc4Cl)-c4c(C)ccn(C)c4=O)c3c2)n1 |(56.02,-27.64,;54.69,-28.42,;54.7,-29.96,;56.04,-30.73,;56.05,-32.27,;54.71,-33.04,;53.39,-32.27,;52.05,-33.04,;50.72,-32.27,;50.71,-30.72,;49.38,-29.96,;48.05,-30.74,;46.59,-30.26,;45.68,-31.5,;44.14,-31.5,;46.58,-32.75,;46.11,-34.21,;44.6,-34.53,;44.12,-35.99,;45.15,-37.14,;46.66,-36.81,;47.14,-35.35,;48.64,-35.03,;42.63,-36.31,;42.15,-37.77,;43.18,-38.92,;40.64,-38.08,;39.61,-36.93,;40.1,-35.47,;39.07,-34.32,;41.6,-35.16,;42.08,-33.69,;48.05,-32.27,;49.38,-33.05,;53.37,-30.73,)|