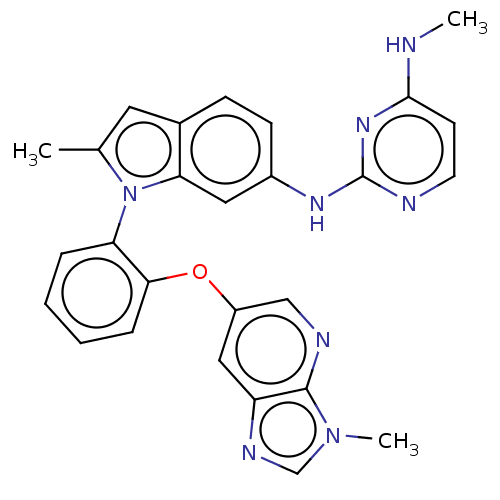
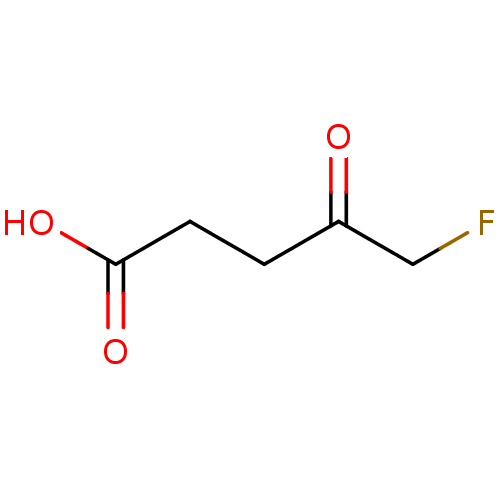
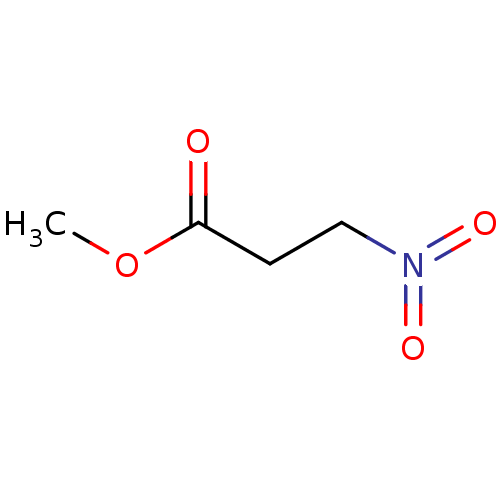
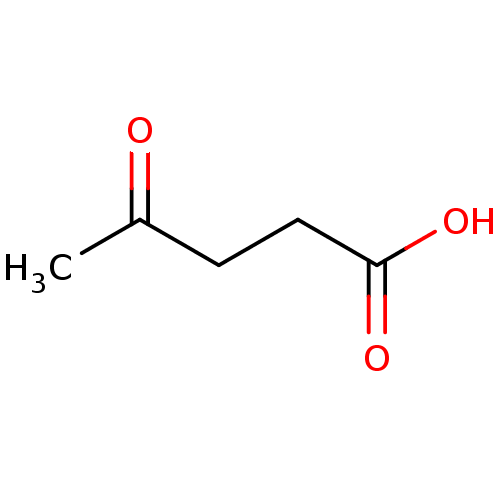
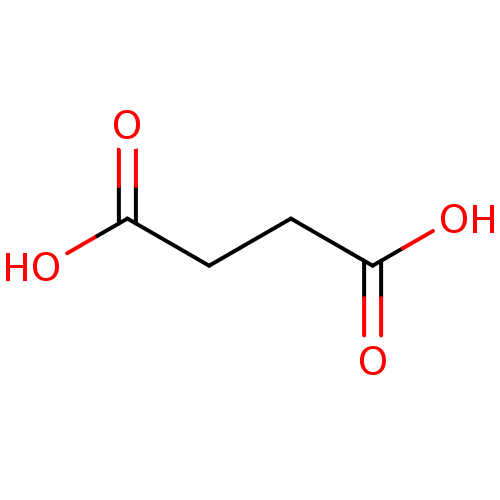
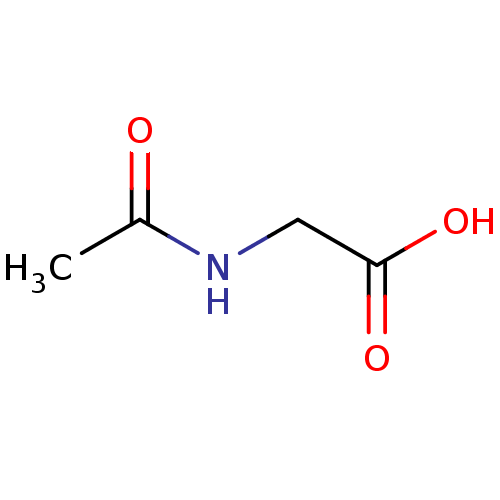
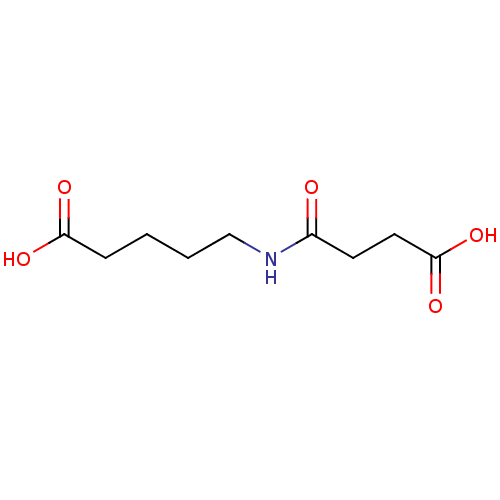
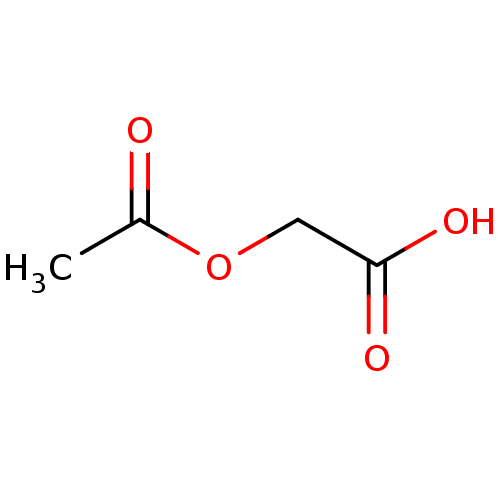
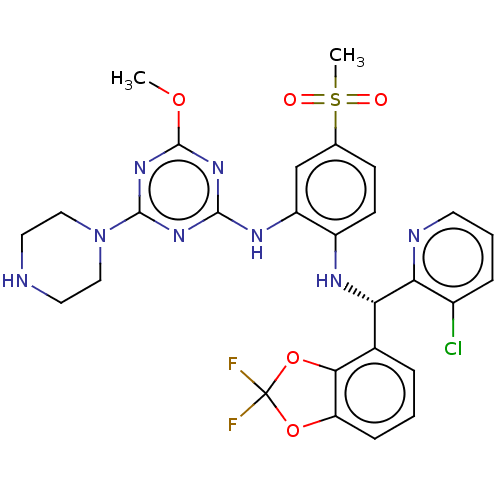
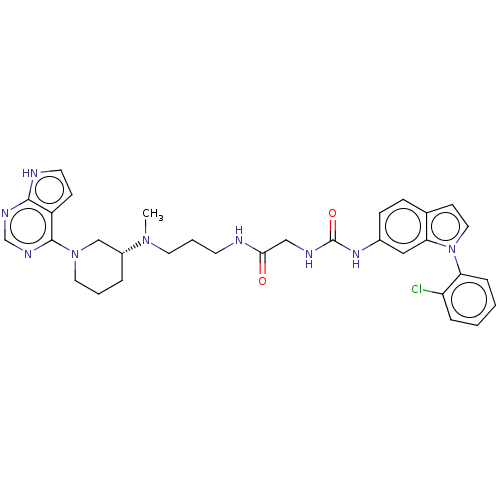
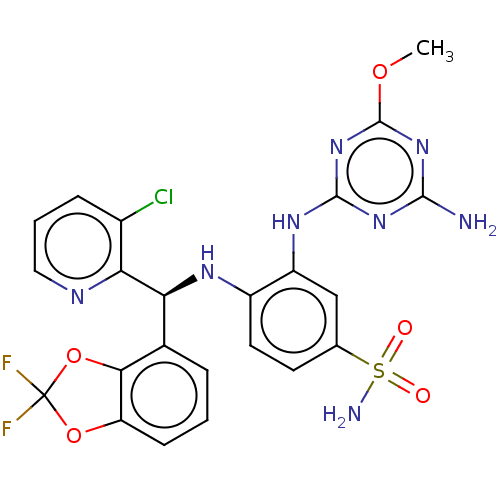
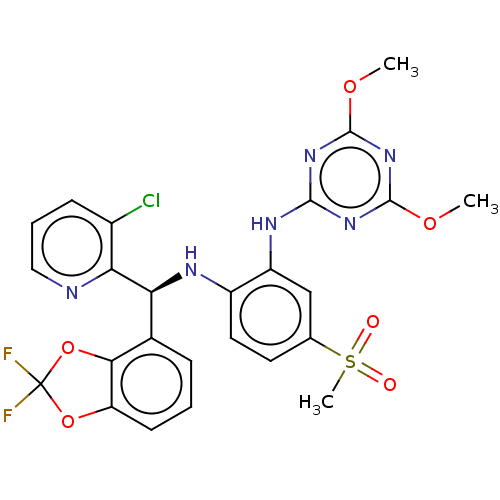
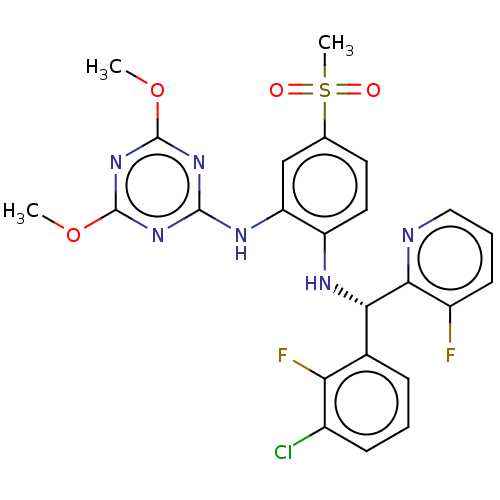
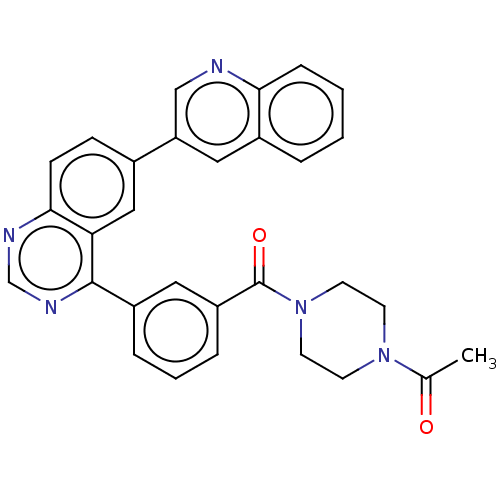
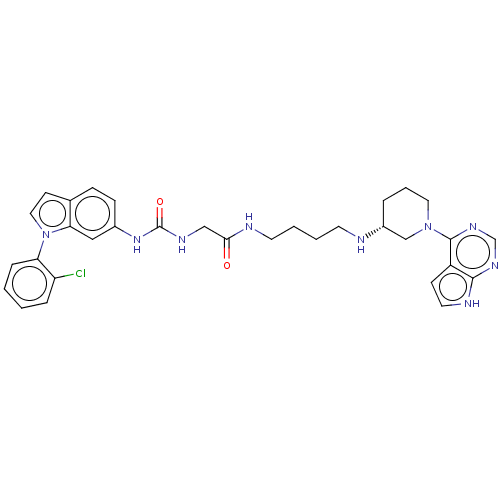
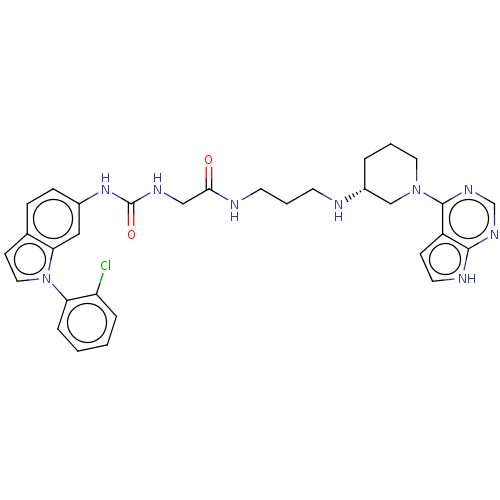
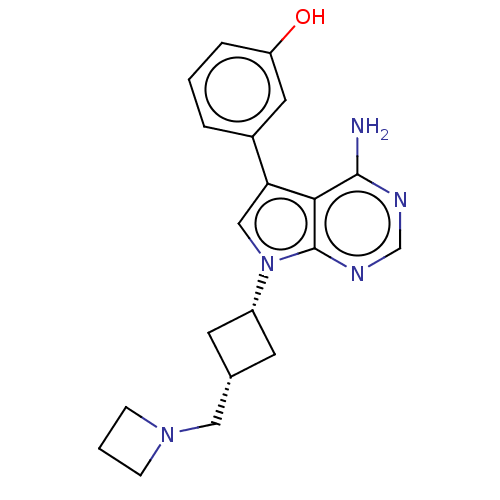
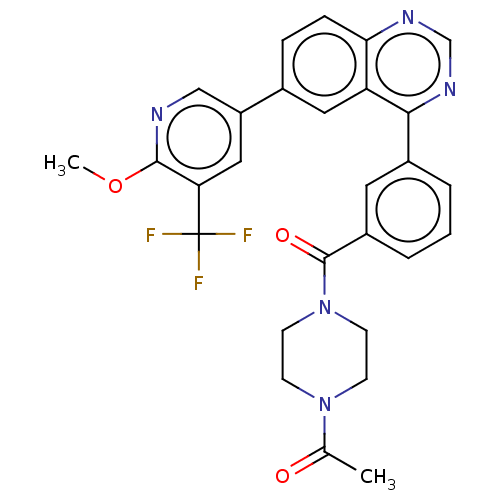
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 0.00200nMAssay Description:Competitive inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by...More data for this Ligand-Target Pair
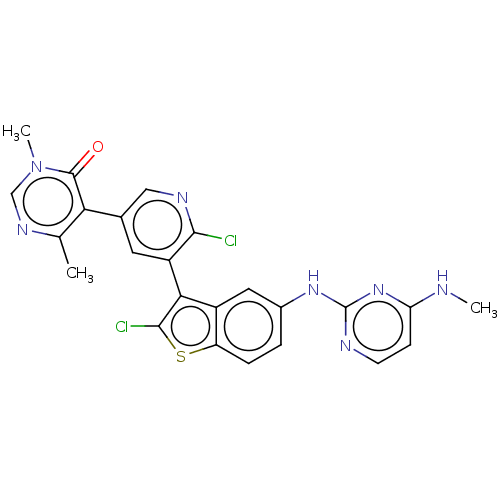
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 0.0120nMAssay Description:Competitive inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by...More data for this Ligand-Target Pair
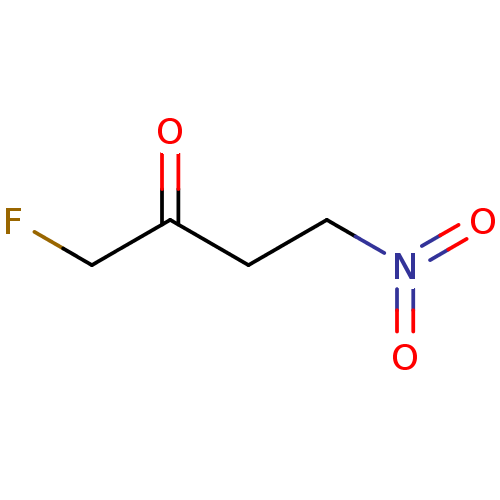
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 0.0800nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
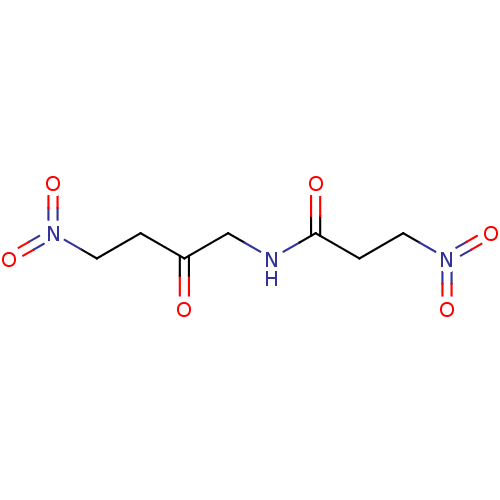
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 0.360nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
Affinity DataKi: 2.00E+4nM ΔG°: -27.9kJ/molepH: 8.1 T: 2°CAssay Description:The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). More data for this Ligand-Target Pair
Affinity DataKi: 2.10E+4nM ΔG°: -27.8kJ/molepH: 8.1 T: 2°CAssay Description:The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). More data for this Ligand-Target Pair
Affinity DataKi: 2.80E+4nM ΔG°: -27.0kJ/molepH: 8.1 T: 2°CAssay Description:The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). More data for this Ligand-Target Pair
Affinity DataKi: 3.00E+4nM ΔG°: -26.9kJ/molepH: 8.1 T: 2°CAssay Description:The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). More data for this Ligand-Target Pair
Affinity DataKi: 8.50E+4nM ΔG°: -24.2kJ/molepH: 8.1 T: 2°CAssay Description:The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). More data for this Ligand-Target Pair
Affinity DataKi: 2.43E+5nM ΔG°: -21.5kJ/molepH: 8.1 T: 2°CAssay Description:The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). More data for this Ligand-Target Pair
Affinity DataKi: 1.88E+6nM ΔG°: -16.2kJ/molepH: 8.1 T: 2°CAssay Description:The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). More data for this Ligand-Target Pair
Affinity DataKi: 2.20E+6nM ΔG°: -15.8kJ/molepH: 8.1 T: 2°CAssay Description:The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). More data for this Ligand-Target Pair
Affinity DataKi: 4.90E+6nM ΔG°: -13.7kJ/molepH: 8.1 T: 2°CAssay Description:The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). More data for this Ligand-Target Pair
Affinity DataKi: 1.25E+7nM ΔG°: -11.3kJ/molepH: 8.1 T: 2°CAssay Description:The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). More data for this Ligand-Target Pair
Affinity DataKi: 1.82E+7nM ΔG°: -10.3kJ/molepH: 8.1 T: 2°CAssay Description:The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). More data for this Ligand-Target Pair
Affinity DataKi: 1.82E+7nM ΔG°: -10.3kJ/molepH: 8.1 T: 2°CAssay Description:The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). More data for this Ligand-Target Pair
Affinity DataKi: 1.88E+7nM ΔG°: -10.2kJ/molepH: 8.1 T: 2°CAssay Description:The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). More data for this Ligand-Target Pair
Affinity DataKi: 2.29E+7nM ΔG°: -9.74kJ/molepH: 8.1 T: 2°CAssay Description:The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). More data for this Ligand-Target Pair
Affinity DataKi: 2.45E+7nM ΔG°: -9.56kJ/molepH: 8.1 T: 2°CAssay Description:The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). More data for this Ligand-Target Pair
Affinity DataKi: 3.64E+7nM ΔG°: -8.54kJ/molepH: 8.1 T: 2°CAssay Description:The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). More data for this Ligand-Target Pair
Affinity DataKi: 3.84E+7nM ΔG°: -8.41kJ/molepH: 8.1 T: 2°CAssay Description:The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). More data for this Ligand-Target Pair
Affinity DataKi: 4.49E+7nM ΔG°: -8.00kJ/molepH: 8.1 T: 2°CAssay Description:The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). More data for this Ligand-Target Pair
Affinity DataKi: >1.50E+8nM ΔG°: >-4.89kJ/molepH: 8.1 T: 2°CAssay Description:The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). More data for this Ligand-Target Pair
Affinity DataKi: 2.23E+8nM ΔG°: -3.87kJ/molepH: 8.1 T: 2°CAssay Description:The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: <0.100nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: <0.100nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.110nMAssay Description:Inhibition of 0.05 nM DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosome as substrate and [3H]SAM as co-factor preincubated for...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.150nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.170nMAssay Description:Inhibition of 0.05 nM DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosome as substrate and [3H]SAM as co-factor preincubated for...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.190nMAssay Description:Inhibition of 0.05 nM DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosome as substrate and [3H]SAM as co-factor preincubated for...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.590nMAssay Description:Inhibition of 0.05 nM DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosome as substrate and [3H]SAM as co-factor preincubated for...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human aromatase coexpressed with P450 reductase by fluorimetryMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase mTOR(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.90nMAssay Description:Displacement of Alexa Fluor labelled Tracer-314 from human N-terminal GST-tagged mTOR incubated for 1 hr by TR-FRET assayMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:Inhibition of 0.05 nM DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosome as substrate and [3H]SAM as co-factor preincubated for...More data for this Ligand-Target Pair
TargetInsulin-like growth factor 1 receptor(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.80nMAssay Description:Inhibition of full length IGF-1 receptor (unknown origin) autophosphorylation transfected in HEK293 cells pretreated for 60 mins followed by IGF-1 st...More data for this Ligand-Target Pair
TargetInsulin-like growth factor 1 receptor(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.80nMAssay Description:Inhibition of full length IGF-1 receptor (unknown origin) autophosphorylation transfected in HEK293 cells pretreated for 60 mins followed by IGF-1 st...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of PI3Kdelta (unknown origin) assessed as reduction in ADP formation using phosphatidyl inositol as substrate after 30 to 60 mins by TR-FR...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of DOT1L in human HeLa cells assessed as reduction in H3K79me2 level after 72 hrs by ELISAMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.90nMAssay Description:Inhibition of 0.5 nM DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosome as substrate and [3H]SAM as co-factor preincubated for ...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
TargetInsulin-like growth factor 1 receptor(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4.30nMAssay Description:Inhibition of IGF-1 receptor (unknown origin) in presence of [gamma33P]ATPMore data for this Ligand-Target Pair
TargetInsulin-like growth factor 1 receptor(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: <4.60nMAssay Description:Inhibition of full length IGF-1 receptor (unknown origin) transfected in Ba/F3 cells assessed as cell proliferationMore data for this Ligand-Target Pair
TargetEphrin type-B receptor 4(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of EphB4 (unknown origin) in presence of [gamma33P]ATPMore data for this Ligand-Target Pair
TargetEphrin type-B receptor 4(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of EphB4 receptor (unknown origin)More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of PI3Kdelta (unknown origin) assessed as reduction in ADP formation using phosphatidyl inositol as substrate after 30 to 60 mins by TR-FR...More data for this Ligand-Target Pair

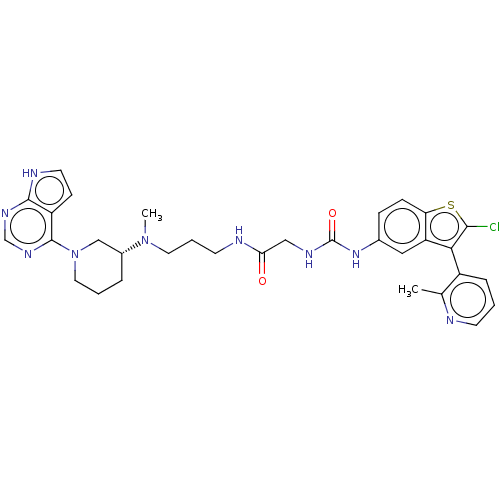
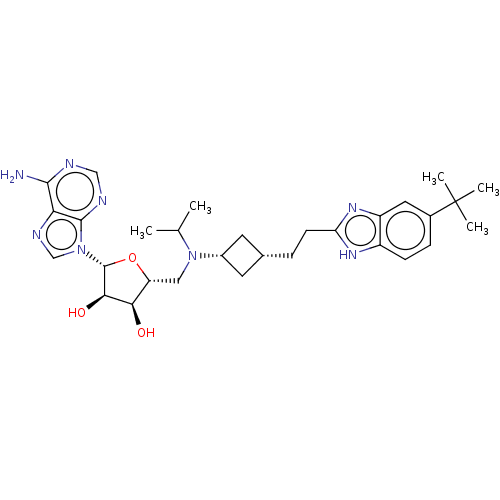
 3D Structure (crystal)
3D Structure (crystal)