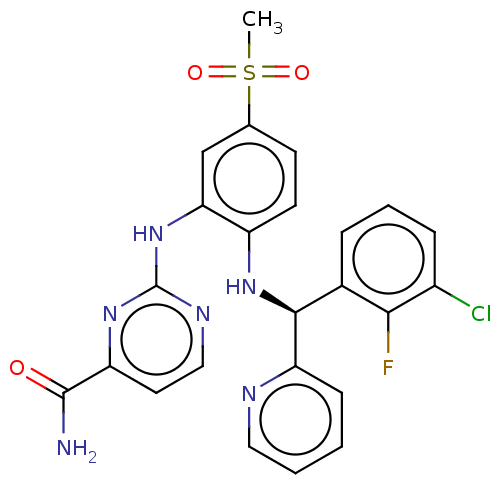
BDBM50529549 CHEMBL4448208
SMILES CS(=O)(=O)c1ccc(N[C@H](c2ccccn2)c2cccc(Cl)c2F)c(Nc2nccc(n2)C(N)=O)c1
InChI Key InChIKey=KYDPLBKTMICGNE-QFIPXVFZSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50529549
Found 2 hits for monomerid = 50529549
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:Inhibition of 0.05 nM DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosome as substrate and [3H]SAM as co-factor preincubated for...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.90nMAssay Description:Inhibition of 0.5 nM DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosome as substrate and [3H]SAM as co-factor preincubated for ...More data for this Ligand-Target Pair