Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Lysine-specific demethylase 4B
Ligand
BDBM50598660
Substrate
n/a
Meas. Tech.
ChEMBL_2227676 (CHEMBL5141189)
IC50
33±n/a nM
Citation
 Wu, Q; Young, B; Wang, Y; Davidoff, AM; Rankovic, Z; Yang, J Recent Advances with KDM4 Inhibitors and Potential Applications. J Med Chem 65:9564-9579 (2022) [PubMed]
Wu, Q; Young, B; Wang, Y; Davidoff, AM; Rankovic, Z; Yang, J Recent Advances with KDM4 Inhibitors and Potential Applications. J Med Chem 65:9564-9579 (2022) [PubMed] More Info.:
Target
Name:
Lysine-specific demethylase 4B
Synonyms:
JHDM3B | JMJD2B | JmjC domain-containing histone demethylation protein 3B | Jumonji domain-containing protein 2B | KDM4B | KDM4B_HUMAN | KIAA0876
Type:
PROTEIN
Mol. Mass.:
121904.33
Organism:
Homo sapiens (Human)
Description:
ChEMBL_109446
Residue:
1096
Sequence:
MGSEDHGAQNPSCKIMTFRPTMEEFKDFNKYVAYIESQGAHRAGLAKIIPPKEWKPRQTYDDIDDVVIPAPIQQVVTGQSGLFTQYNIQKKAMTVGEYRRLANSEKYCTPRHQDFDDLERKYWKNLTFVSPIYGADISGSLYDDDVAQWNIGSLRTILDMVERECGTIIEGVNTPYLYFGMWKTTFAWHTEDMDLYSINYLHFGEPKSWYAIPPEHGKRLERLAIGFFPGSSQGCDAFLRHKMTLISPIILKKYGIPFSRITQEAGEFMITFPYGYHAGFNHGFNCAESTNFATLRWIDYGKVATQCTCRKDMVKISMDVFVRILQPERYELWKQGKDLTVLDHTRPTALTSPELSSWSASRASLKAKLLRRSHRKRSQPKKPKPEDPKFPGEGTAGAALLEEAGGSVKEEAGPEVDPEEEEEEPQPLPHGREAEGAEEDGRGKLRPTKAKSERKKKSFGLLPPQLPPPPAHFPSEEALWLPSPLEPPVLGPGPAAMEESPLPAPLNVVPPEVPSEELEAKPRPIIPMLYVVPRPGKAAFNQEHVSCQQAFEHFAQKGPTWKEPVSPMELTGPEDGAASSGAGRMETKARAGEGQAPSTFSKLKMEIKKSRRHPLGRPPTRSPLSVVKQEASSDEEASPFSGEEDVSDPDALRPLLSLQWKNRAASFQAERKFNAAAARTEPYCAICTLFYPYCQALQTEKEAPIASLGKGCPATLPSKSRQKTRPLIPEMCFTSGGENTEPLPANSYIGDDGTSPLIACGKCCLQVHASCYGIRPELVNEGWTCSRCAAHAWTAECCLCNLRGGALQMTTDRRWIHVICAIAVPEARFLNVIERHPVDISAIPEQRWKLKCVYCRKRMKKVSGACIQCSYEHCSTSFHVTCAHAAGVLMEPDDWPYVVSITCLKHKSGGHAVQLLRAVSLGQVVITKNRNGLYYRCRVIGAASQTCYEVNFDDGSYSDNLYPESITSRDCVQLGPPSEGELVELRWTDGNLYKAKFISSVTSHIYQVEFEDGSQLTVKRGDIFTLEEELPKRVRSRLSLSTGAPQEPAFSGEEAKAAKRPRVGTPLATEDSGRSQDYVAFVESLLQVQGRPGAPF
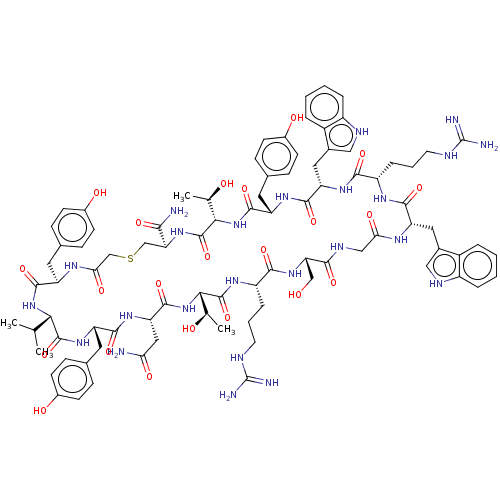
Inhibitor
Name:
BDBM50598660
Synonyms:
CHEMBL5203308
Type:
Small organic molecule
Emp. Form.:
C88H116N24O22S
Mol. Mass.:
1894.075
SMILES:
CC(C)[C@@H]1NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)CSC[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)[C@@H](C)O)[C@@H](C)O)C(N)=O |r|