Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 2C9
Ligand
BDBM50406134
Substrate
n/a
Meas. Tech.
ChEMBL_2266236
IC50
>50000±n/a nM
Citation
 Roecker, AJ; Schirripa, KM; Loughran, HM; Tong, L; Liang, T; Fillgrove, KL; Kuo, Y; Bleasby, K; Collier, H; Altman, MD; Ford, MC; Drolet, RE; Cosden, M; Jinn, S; Hatcher, NG; Yao, L; Kandebo, M; Vardigan, JD; Flick, RB; Liu, X; Minnick, C; Price, LA; Watt, ML; Lemaire, W; Burlein, C; Adam, GC; Austin, LA; Marcus, JN; Smith, SM; Fraley, ME Pyrazole Ureas as Low Dose, CNS Penetrant Glucosylceramide Synthase Inhibitors for the Treatment of Parkinson's Disease. ACS Med Chem Lett 14:146-155 (2023) [PubMed]
Roecker, AJ; Schirripa, KM; Loughran, HM; Tong, L; Liang, T; Fillgrove, KL; Kuo, Y; Bleasby, K; Collier, H; Altman, MD; Ford, MC; Drolet, RE; Cosden, M; Jinn, S; Hatcher, NG; Yao, L; Kandebo, M; Vardigan, JD; Flick, RB; Liu, X; Minnick, C; Price, LA; Watt, ML; Lemaire, W; Burlein, C; Adam, GC; Austin, LA; Marcus, JN; Smith, SM; Fraley, ME Pyrazole Ureas as Low Dose, CNS Penetrant Glucosylceramide Synthase Inhibitors for the Treatment of Parkinson's Disease. ACS Med Chem Lett 14:146-155 (2023) [PubMed] More Info.:
Target
Name:
Cytochrome P450 2C9
Synonyms:
(R)-limonene 6-monooxygenase | (S)-limonene 6-monooxygenase | CP2C9_HUMAN | CYP2C10 | CYP2C9 | CYPIIC9 | Cytochrome P450 2C9 (CYP2C9 ) | Cytochrome P450 2C9 (CYP2C9) | P-450MP | P450 MP-4/MP-8 | P450 PB-1 | S-mephenytoin 4-hydroxylase
Type:
Enzyme
Mol. Mass.:
55636.33
Organism:
Homo sapiens (Human)
Description:
P11712
Residue:
490
Sequence:
MDSLVVLVLCLSCLLLLSLWRQSSGRGKLPPGPTPLPVIGNILQIGIKDISKSLTNLSKVYGPVFTLYFGLKPIVVLHGYEAVKEALIDLGEEFSGRGIFPLAERANRGFGIVFSNGKKWKEIRRFSLMTLRNFGMGKRSIEDRVQEEARCLVEELRKTKASPCDPTFILGCAPCNVICSIIFHKRFDYKDQQFLNLMEKLNENIKILSSPWIQICNNFSPIIDYFPGTHNKLLKNVAFMKSYILEKVKEHQESMDMNNPQDFIDCFLMKMEKEKHNQPSEFTIESLENTAVDLFGAGTETTSTTLRYALLLLLKHPEVTAKVQEEIERVIGRNRSPCMQDRSHMPYTDAVVHEVQRYIDLLPTSLPHAVTCDIKFRNYLIPKGTTILISLTSVLHDNKEFPNPEMFDPHHFLDEGGNFKKSKYFMPFSAGKRICVGEALAGMELFLFLTSILQNFNLKSLVDPKNLDTTPVVNGFASVPPFYQLCFIPV
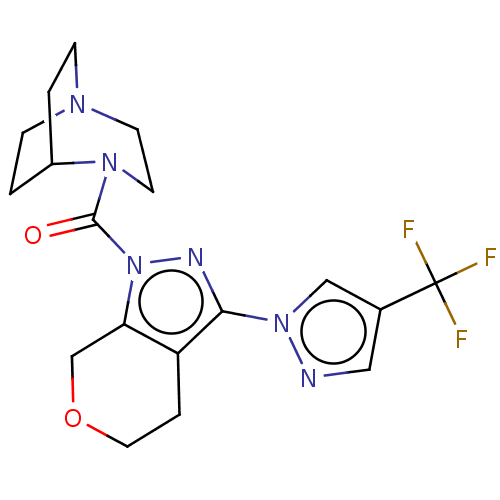
Inhibitor
Name:
BDBM50406134
Synonyms:
CHEMBL5275403
Type:
Small organic molecule
Emp. Form.:
C18H22ClF3N6O2
Mol. Mass.:
446.854
SMILES:
Cl.FC(F)(F)c1cnn(c1)-c1nn(C(=O)N2CCN3CCC2CC3)c2COCCc12 |(3.57,-1.5,;.59,-6.29,;-.5,-5.2,;-1.99,-5.6,;-.9,-6.69,;-.1,-3.71,;1.25,-3.18,;1.25,-1.61,;-.24,-1.21,;-1.06,-2.5,;-.64,.28,;.28,1.56,;-.64,2.79,;-.18,4.23,;-1.26,5.36,;1.31,4.59,;2.28,3.36,;3.82,3.36,;4.8,4.54,;3.46,4.18,;2.23,4.79,;1.72,6.08,;3.1,6.69,;4.49,6.02,;-2.13,2.33,;-3.46,3.1,;-4.8,2.33,;-4.8,.79,;-3.46,.02,;-2.13,.79,)|