Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Genome polyprotein
Ligand
BDBM50301902
Substrate
n/a
Meas. Tech.
ChEMBL_598467 (CHEMBL1039991)
IC50
3±n/a nM
Citation
 de Vicente, J; Hendricks, RT; Smith, DB; Fell, JB; Fischer, J; Spencer, SR; Stengel, PJ; Mohr, P; Robinson, JE; Blake, JF; Hilgenkamp, RK; Yee, C; Zhao, J; Elworthy, TR; Tracy, J; Chin, E; Li, J; Lui, A; Wang, B; Oshiro, C; Harris, SF; Ghate, M; Leveque, VJ; Najera, I; Le Pogam, S; Rajyaguru, S; Ao-Ieong, G; Alexandrova, L; Fitch, B; Brandl, M; Masjedizadeh, M; Wu, SY; de Keczer, S; Voronin, T Non-nucleoside inhibitors of HCV polymerase NS5B. Part 3: synthesis and optimization studies of benzothiazine-substituted tetramic acids. Bioorg Med Chem Lett 19:5648-51 (2009) [PubMed] Article
de Vicente, J; Hendricks, RT; Smith, DB; Fell, JB; Fischer, J; Spencer, SR; Stengel, PJ; Mohr, P; Robinson, JE; Blake, JF; Hilgenkamp, RK; Yee, C; Zhao, J; Elworthy, TR; Tracy, J; Chin, E; Li, J; Lui, A; Wang, B; Oshiro, C; Harris, SF; Ghate, M; Leveque, VJ; Najera, I; Le Pogam, S; Rajyaguru, S; Ao-Ieong, G; Alexandrova, L; Fitch, B; Brandl, M; Masjedizadeh, M; Wu, SY; de Keczer, S; Voronin, T Non-nucleoside inhibitors of HCV polymerase NS5B. Part 3: synthesis and optimization studies of benzothiazine-substituted tetramic acids. Bioorg Med Chem Lett 19:5648-51 (2009) [PubMed] Article More Info.:
Target
Name:
Genome polyprotein
Synonyms:
Genome polyprotein | POLG_HCVCO | RNA polymerase (NS5B)
Type:
Enzyme
Mol. Mass.:
326966.11
Organism:
Hepatitis C virus (HCV)
Description:
Q9WMX2
Residue:
3010
Sequence:
MSTNPKPQRKTKRNTNRRPQDVKFPGGGQIVGGVYLLPRRGPRLGVRATRKTSERSQPRGRRQPIPKARQPEGRAWAQPGYPWPLYGNEGLGWAGWLLSPRGSRPSWGPTDPRRRSRNLGKVIDTLTCGFADLMGYIPLVGAPLGGAARALAHGVRVLEDGVNYATGNLPGCSFSIFLLALLSCLTIPASAYEVRNVSGVYHVTNDCSNASIVYEAADMIMHTPGCVPCVRENNSSRCWVALTPTLAARNASVPTTTIRRHVDLLVGAAALCSAMYVGDLCGSVFLVAQLFTFSPRRHETVQDCNCSIYPGHVTGHRMAWDMMMNWSPTAALVVSQLLRIPQAVVDMVAGAHWGVLAGLAYYSMVGNWAKVLIVMLLFAGVDGGTYVTGGTMAKNTLGITSLFSPGSSQKIQLVNTNGSWHINRTALNCNDSLNTGFLAALFYVHKFNSSGCPERMASCSPIDAFAQGWGPITYNESHSSDQRPYCWHYAPRPCGIVPAAQVCGPVYCFTPSPVVVGTTDRFGVPTYSWGENETDVLLLNNTRPPQGNWFGCTWMNSTGFTKTCGGPPCNIGGIGNKTLTCPTDCFRKHPEATYTKCGSGPWLTPRCLVHYPYRLWHYPCTVNFTIFKVRMYVGGVEHRLEAACNWTRGERCNLEDRDRSELSPLLLSTTEWQVLPCSFTTLPALSTGLIHLHQNVVDVQYLYGIGSAVVSFAIKWEYVLLLFLLLADARVCACLWMMLLIAQAEAALENLVVLNAASVAGAHGILSFLVFFCAAWYIKGRLVPGAAYALYGVWPLLLLLLALPPRAYAMDREMAASCGGAVFVGLILLTLSPHYKLFLARLIWWLQYFITRAEAHLQVWIPPLNVRGGRDAVILLTCAIHPELIFTITKILLAILGPLMVLQAGITKVPYFVRAHGLIRACMLVRKVAGGHYVQMALMKLAALTGTYVYDHLTPLRDWAHAGLRDLAVAVEPVVFSDMETKVITWGADTAACGDIILGLPVSARRGREIHLGPADSLEGQGWRLLAPITAYSQQTRGLLGCIITSLTGRDRNQVEGEVQVVSTATQSFLATCVNGVCWTVYHGAGSKTLAGPKGPITQMYTNVDQDLVGWQAPPGARSLTPCTCGSSDLYLVTRHADVIPVRRRGDSRGSLLSPRPVSYLKGSSGGPLLCPSGHAVGIFRAAVCTRGVAKAVDFVPVESMETTMRSPVFTDNSSPPAVPQTFQVAHLHAPTGSGKSTKVPAAYAAQGYKVLVLNPSVAATLGFGAYMSKAHGIDPNIRTGVRTITTGAPITYSTYGKFLADGGCSGGAYDIIICDECHSTDSTTILGIGTVLDQAETAGARLVVLATATPPGSVTVPHPNIEEVALSSTGEIPFYGKAIPIETIKGGRHLIFCHSKKKCDELAAKLSGLGLNAVAYYRGLDVSVIPTSGDVIVVATDALMTGFTGDFDSVIDCNTCVTQTVDFSLDPTFTIETTTVPQDAVSRSQRRGRTGRGRMGIYRFVTPGERPSGMFDSSVLCECYDAGCAWYELTPAETSVRLRAYLNTPGLPVCQDHLEFWESVFTGLTHIDAHFLSQTKQAGDNFPYLVAYQATVCARAQAPPPSWDQMWKCLIRLKPTLHGPTPLLYRLGAVQNEVTTTHPITKYIMACMSADLEVVTSTWVLVGGVLAALAAYCLTTGSVVIVGRIILSGKPAIIPDREVLYREFDEMEECASHLPYIEQGMQLAEQFKQKAIGLLQTATKQAEAAAPVVESKWRTLEAFWAKHMWNFISGIQYLAGLSTLPGNPAIASLMAFTASITSPLTTQHTLLFNILGGWVAAQLAPPSAASAFVGAGIAGAAVGSIGLGKVLVDILAGYGAGVAGALVAFKVMSGEMPSTEDLVNLLPAILSPGALVVGVVCAAILRRHVGPGEGAVQWMNRLIAFASRGNHVSPTHYVPESDAAARVTQILSSLTITQLLKRLHQWINEDCSTPCSGSWLRDVWDWICTVLTDFKTWLQSKLLPRLPGVPFFSCQRGYKGVWRGDGIMQTTCPCGAQITGHVKNGSMRIVGPRTCSNTWHGTFPINAYTTGPCTPSPAPNYSRALWRVAAEEYVEVTRVGDFHYVTGMTTDNVKCPCQVPAPEFFTEVDGVRLHRYAPACKPLLREEVTFLVGLNQYLVGSQLPCEPEPDVAVLTSMLTDPSHITAETAKRRLARGSPPSLASSSASQLSAPSLKATCTTRHDSPDADLIEANLLWRQEMGGNITRVESENKVVILDSFEPLQAEEDEREVSVPAEILRRSRKFPRAMPIWARPDYNPPLLESWKDPDYVPPVVHGCPLPPAKAPPIPPPRRKRTVVLSESTVSSALAELATKTFGSSESSAVDSGTATASPDQPSDDGDAGSDVESYSSMPPLEGEPGDPDLSDGSWSTVSEEASEDVVCCSMSYTWTGALITPCAAEETKLPINALSNSLLRHHNLVYATTSRSASLRQKKVTFDRLQVLDDHYRDVLKEMKAKASTVKAKLLSVEEACKLTPPHSARSKFGYGAKDVRNLSSKAVNHIRSVWKDLLEDTETPIDTTIMAKNEVFCVQPEKGGRKPARLIVFPDLGVRVCEKMALYDVVSTLPQAVMGSSYGFQYSPGQRVEFLVNAWKAKKCPMGFAYDTRCFDSTVTENDIRVEESIYQCCDLAPEARQAIRSLTERLYIGGPLTNSKGQNCGYRRCRASGVLTTSCGNTLTCYLKAAAACRAAKLQDCTMLVCGDDLVVICESAGTQEDEASLRAFTEAMTRYSAPPGDPPKPEYDLELITSCSSNVSVAHDASGKRVYYLTRDPTTPLARAAWETARHTPVNSWLGNIIMYAPTLWARMILMTHFFSILLAQEQLEKALDCQIYGACYSIEPLDLPQIIQRLHGLSAFSLHSYSPGEINRVASCLRKLGVPPLRVWRHRARSVRARLLSQGGRAATCGKYLFNWAVRTKLKLTPIPAASQLDLSSWFVAGYSGGDIYHSLSRARPRWFMWCLLLLSVGVGIYLLPNR
Inhibitor
Name:
BDBM50301902
Synonyms:
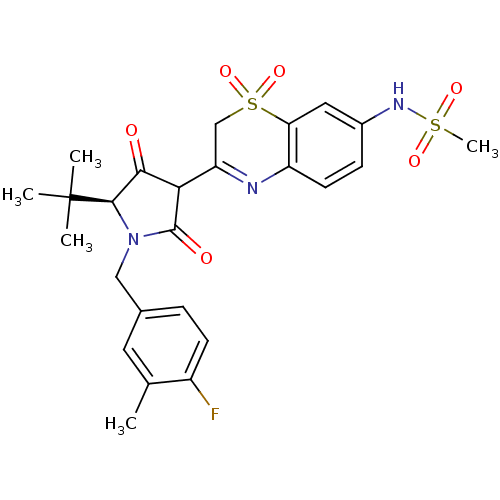
CHEMBL571825 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-3-methyl-benzyl)-4-hydroxy-2-oxo-2,5-dihydro-1H-pyrrol-3-yl]-1,1-dioxo-1,4-dihydro-1lambda*6*-benzo[1,4]thiazin-7-yl}-methanesulfonamide
Type:
Small organic molecule
Emp. Form.:
C25H28FN3O6S2
Mol. Mass.:
549.635
SMILES:
Cc1cc(CN2[C@H](C(=O)C(C2=O)C2=Nc3ccc(NS(C)(=O)=O)cc3S(=O)(=O)C2)C(C)(C)C)ccc1F |r,t:13|