Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 3A4
Ligand
BDBM50310778
Substrate
n/a
Meas. Tech.
ChEMBL_621465 (CHEMBL1100026)
IC50
91±n/a nM
Citation
 Flentge, CA; Randolph, JT; Huang, PP; Klein, LL; Marsh, KC; Harlan, JE; Kempf, DJ Synthesis and evaluation of inhibitors of cytochrome P450 3A (CYP3A) for pharmacokinetic enhancement of drugs. Bioorg Med Chem Lett 19:5444-8 (2009) [PubMed] Article
Flentge, CA; Randolph, JT; Huang, PP; Klein, LL; Marsh, KC; Harlan, JE; Kempf, DJ Synthesis and evaluation of inhibitors of cytochrome P450 3A (CYP3A) for pharmacokinetic enhancement of drugs. Bioorg Med Chem Lett 19:5444-8 (2009) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 3A4
Synonyms:
Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase
Type:
Enzyme
Mol. Mass.:
57349.57
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
503
Sequence:
MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMFDMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISIAEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYSMDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICVFPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSIIFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVVNETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFSKKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLGGLLQPEKPVVLKVESRDGTVSGA
Inhibitor
Name:
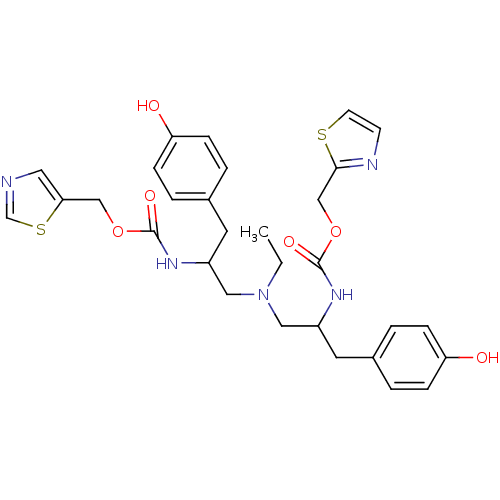
BDBM50310778
Synonyms:
CHEMBL1078122 | N-[(S)-2-(thiazol-5-ylmethoxycarbonylamino)-3-(4-phenylmethoxyphenyl)propyl]-N-[2-(thiazol-5-ylmethoxycarbonylamino)-3-(4-phenylmethoxyphenyl)propyl]-ethylamine
Type:
Small organic molecule
Emp. Form.:
C30H35N5O6S2
Mol. Mass.:
625.759
SMILES:
CCN(CC(Cc1ccc(O)cc1)NC(=O)OCc1cncs1)CC(Cc1ccc(O)cc1)NC(=O)OCc1nccs1