Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Lactoylglutathione lyase
Ligand
BDBM50295287
Substrate
n/a
Meas. Tech.
ChEMBL_754309 (CHEMBL1799085)
Ki
87800±n/a nM
Citation
More Info.:
Target
Name:
Lactoylglutathione lyase
Synonyms:
Aldoketomutase | GLO1 | Glx I | Glyoxalase 1 (GLO1) | Glyoxalase I | Ketone-aldehyde mutase | LGUL_HUMAN | Methylglyoxalase | S-D-lactoylglutathione methylglyoxal lyase
Type:
Enzyme
Mol. Mass.:
20772.95
Organism:
Homo sapiens (Human)
Description:
Q04760
Residue:
184
Sequence:
MAEPQPPSGGLTDEAALSCCSDADPSTKDFLLQQTMLRVKDPKKSLDFYTRVLGMTLIQKCDFPIMKFSLYFLAYEDKNDIPKEKDEKIAWALSRKATLELTHNWGTEDDETQSYHNGNSDPRGFGHIGIAVPDVYSACKRFEELGVKFVKKPDDGKMKGLAFIQDPDGYWIEILNPNKMATLM
Inhibitor
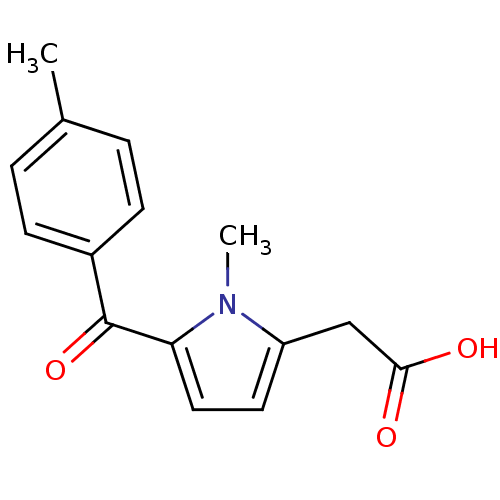
Name:
BDBM50295287
Synonyms:
2-(1-methyl-5-(4-methylbenzoyl)-1H-pyrrol-2-yl)acetic acid | CHEMBL1020 | MCN-2559 | TOLMETIN | Tolectin | [1-Methyl-5-(4-methyl-benzoyl)-1H-pyrrol-2-yl]-acetic acid | [1-Methyl-5-(4-methyl-benzoyl)-1H-pyrrol-2-yl]-acetic acid(tolmetin)
Type:
Small organic molecule
Emp. Form.:
C15H15NO3
Mol. Mass.:
257.2845
SMILES:
Cc1ccc(cc1)C(=O)c1ccc(CC(O)=O)n1C