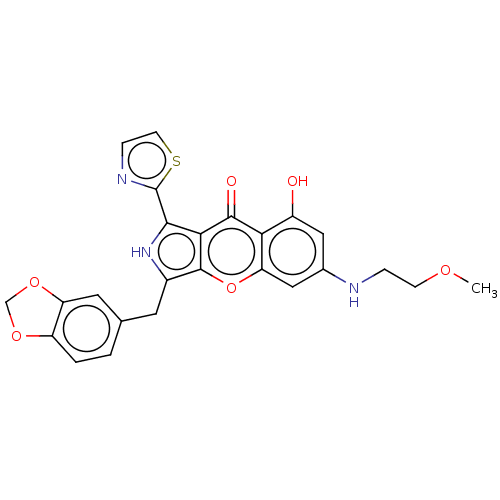
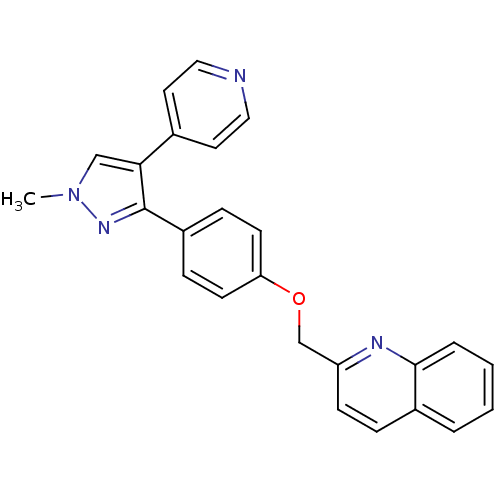
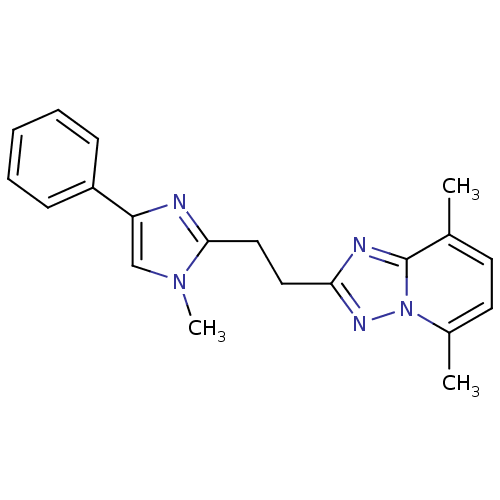
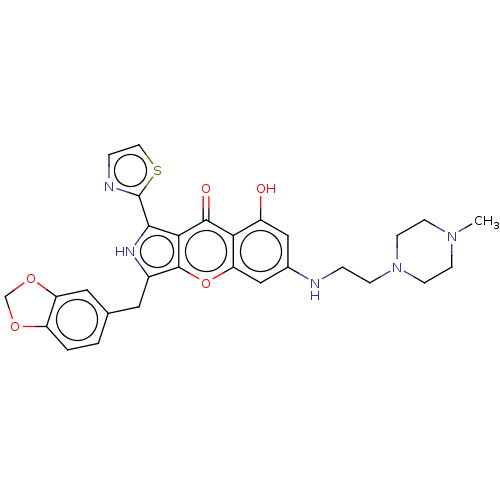
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
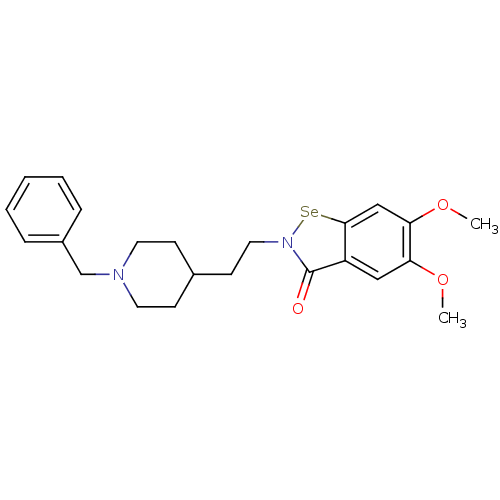
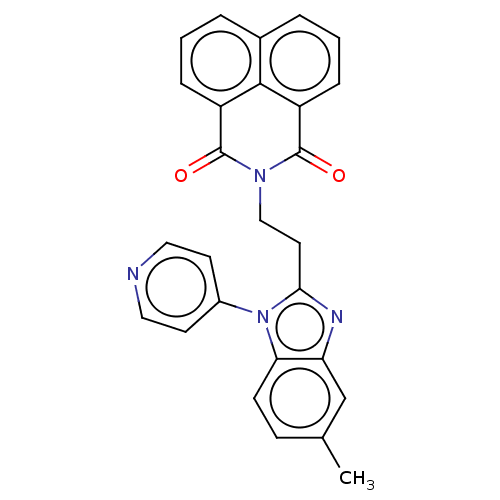
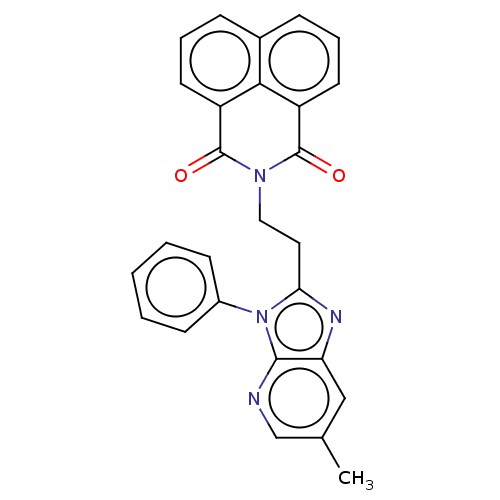
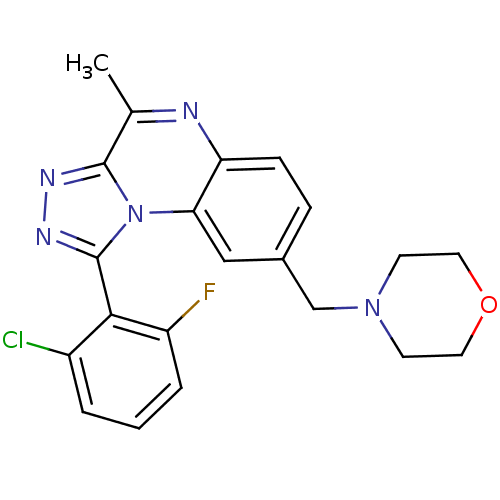
Affinity DataKi: 7nMAssay Description:Binding affinity to PDE9A2 (unknown origin)More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
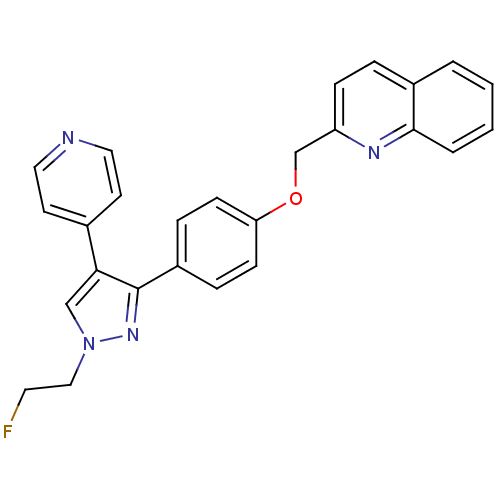
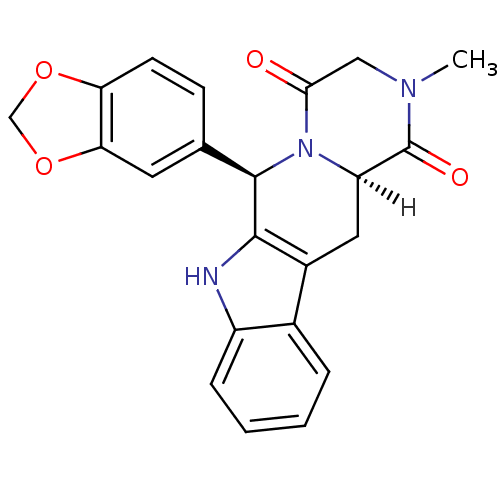
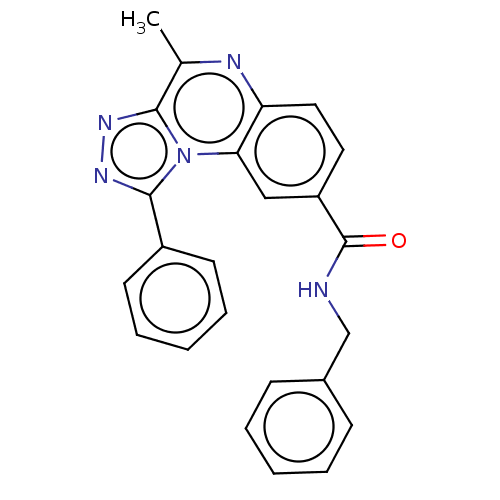
Affinity DataKi: 17nMAssay Description:Competitive inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addition by ...More data for this Ligand-Target Pair
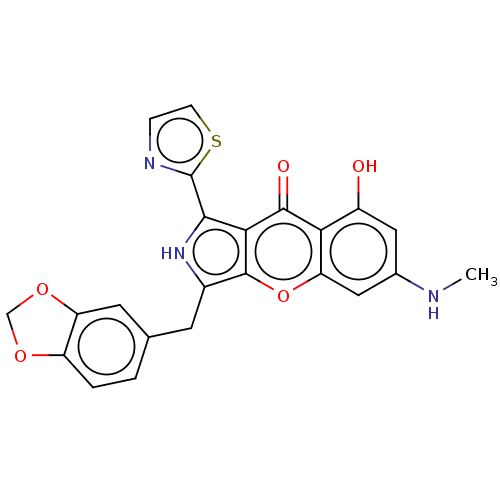
Affinity DataKi: 1.03E+4nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
Affinity DataKi: 1.82E+4nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
Affinity DataKi: 2.44E+4nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
Affinity DataKi: 8.78E+4nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
Affinity DataKi: 3.35E+5nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
Affinity DataKi: 3.83E+5nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
Affinity DataKi: 8.43E+5nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
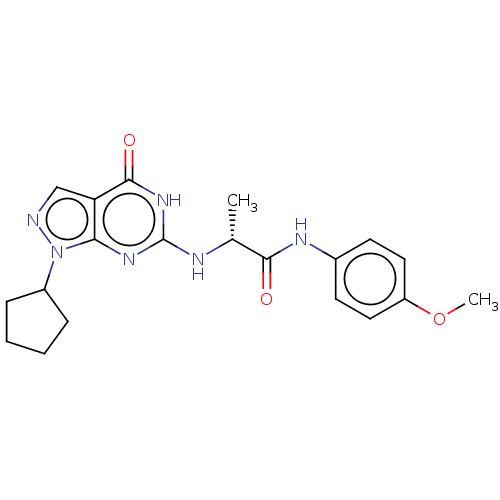
Affinity DataIC50: 0.0600nMAssay Description:Antagonist activity at CCK2 receptor in immature rat stomach assessed as pentagastrin-induced acid secretionMore data for this Ligand-Target Pair
Affinity DataIC50: 0.130nMAssay Description:Antagonist activity at CCK2 receptor in immature rat stomach assessed as pentagastrin-induced acid secretionMore data for this Ligand-Target Pair
Affinity DataIC50: 0.180nMAssay Description:Antagonist activity at CCK2 receptor in immature rat stomach assessed as pentagastrin-induced acid secretionMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibition of human PDE10A2 catalytic domain (446 to 789 residues) expressed in Escherichia coli BL21 cells using [3H]-cGMP as substrate after 15 min...More data for this Ligand-Target Pair
TargetHigh affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B(Homo sapiens (Human))TBA
Affinity DataIC50: 0.300nMAssay Description:Antagonist activity at AT1 receptor assessed inhibition of angiotensin-2-induced contraction of rabbit thoracic aortic ringsMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 0.320nMAssay Description:Inhibition of human PDE5A1 catalytic domain (535 to 860 residues) expressed in Escherichia coli BL21 using 3H-cGMP as substrate after 15 mins by liqu...More data for this Ligand-Target Pair
Affinity DataIC50: 0.370nMAssay Description:Inhibition of human PDE10A2 catalytic domain (446 to 789 residues) expressed in Escherichia coli BL21 cells using [3H]-cGMP as substrate after 15 min...More data for this Ligand-Target Pair
Affinity DataIC50: 0.390nMAssay Description:Inhibition of P-glycoprotein expressed in MDCK-MDR1 cells by calcein AM assayMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 0.390nMAssay Description:Inhibition of human PDE5A1 catalytic domain (535 to 860 residues) expressed in Escherichia coli BL21 using 3H-cGMP as substrate after 15 mins by liqu...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D(Homo sapiens (Human))
National-Local Joint Engineering Laboratory Of Druggability And New Drugs Evaluation
Curated by ChEMBL
National-Local Joint Engineering Laboratory Of Druggability And New Drugs Evaluation
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of PDE4D (unknown origin)More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D(Homo sapiens (Human))
National-Local Joint Engineering Laboratory Of Druggability And New Drugs Evaluation
Curated by ChEMBL
National-Local Joint Engineering Laboratory Of Druggability And New Drugs Evaluation
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of PDE4D2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.440nMAssay Description:Inhibition of human PDE10A2 catalytic domain (446 to 789 residues) expressed in Escherichia coli BL21 cells using [3H]-cGMP as substrate after 15 min...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Antagonist activity at CCK2 receptor in immature rat stomach assessed as pentagastrin-induced acid secretionMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 0.520nMAssay Description:Inhibition of human PDE5A1 catalytic domain (535 to 860 residues) expressed in Escherichia coli BL21 using 3H-cGMP as substrate after 15 mins by liqu...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
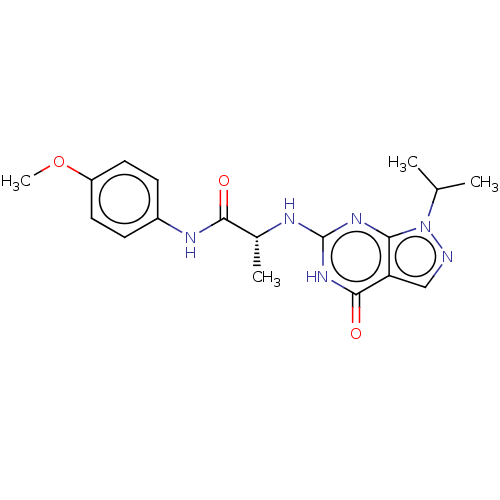
Affinity DataIC50: 0.600nMAssay Description:Inhibition of PDE9A (unknown origin)More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of PDE9A2 catalytic domain (unknown origin) using [3H]-cGMP/[3H]-cAMP as substrate after 15 mins by liquid scintillation counting analysisMore data for this Ligand-Target Pair
TargetHigh affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A(Homo sapiens (Human))TBA
Affinity DataIC50: 0.700nMAssay Description:Antagonist activity at human EP1 receptor expressed in CHO cells receptor by NFTA reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.760nMAssay Description:Inhibition of human PDE10A2 catalytic domain (446 to 789 residues) expressed in Escherichia coli BL21 cells using [3H]-cGMP as substrate after 15 min...More data for this Ligand-Target Pair
Affinity DataIC50: 0.770nMAssay Description:Antagonist activity at CCK2 receptor in immature rat stomach assessed as pentagastrin-induced acid secretionMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 0.790nMAssay Description:Inhibition of human PDE5A1 catalytic domain (535 to 860 residues) expressed in Escherichia coli BL21 using 3H-cGMP as substrate after 15 mins by liqu...More data for this Ligand-Target Pair
Affinity DataIC50: 0.870nMAssay Description:Inhibition of PDE10 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.870nMAssay Description:Inhibition of PDE10 (unknown origin)More data for this Ligand-Target Pair
TargetDual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C(Homo sapiens (Human))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Antagonist activity at AT1 receptor assessed inhibition of angiotensin-2-induced contraction of rabbit thoracic aortic ringsMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Antagonist activity at AT1 receptor assessed inhibition of angiotensin-2-induced contraction of rabbit thoracic aortic ringsMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human PDE10A2 catalytic domain (446 to 789 residues) expressed in Escherichia coli BL21 cells using [3H]-cGMP as substrate after 15 min...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
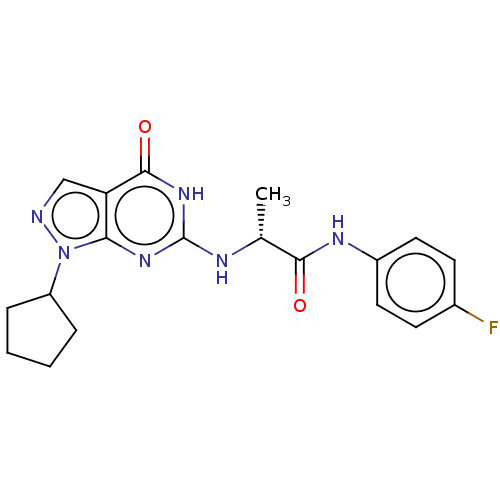
Affinity DataIC50: 1.10nMAssay Description:Inhibition of PDE9A2 catalytic domain (unknown origin) using [3H]-cGMP/[3H]-cAMP as substrate after 15 mins by liquid scintillation counting analysisMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of human PDE5A1 catalytic domain (535 to 860 residues) expressed in Escherichia coli BL21 using 3H-cGMP as substrate after 15 mins by liqu...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of PDE9A2 catalytic domain (unknown origin) using [3H]-cGMP/[3H]-cAMP as substrate after 15 mins by liquid scintillation counting analysisMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of PDE5A (unknown origin)More data for this Ligand-Target Pair
TargetCone cGMP-specific 3',5'-cyclic phosphodiesterase subunit alpha'(Homo sapiens (Human))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of PDE6C catalytic domain (1 to 858 residues) (unknown origin) expressed in Escherichia coli BL21 using 3H-cGMP as substrate after 15 mins...More data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of human PDE5A1 catalytic domain (535 to 860 residues) expressed in Escherichia coli BL21 using 3H-cGMP as substrate after 15 mins by liqu...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Antagonist activity at AT1 receptor assessed inhibition of angiotensin-2-induced contraction of rabbit thoracic aortic ringsMore data for this Ligand-Target Pair
TargetDual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C(Homo sapiens (Human))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Inhibition of human PDE5A1 catalytic domain (535 to 860 residues) expressed in Escherichia coli BL21 using 3H-cGMP as substrate after 15 mins by liqu...More data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Inhibition of human PDE5A1 catalytic domain (535 to 860 residues) expressed in Escherichia coli BL21 using 3H-cGMP as substrate after 15 mins by liqu...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Inhibition of PDE2A (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Inhibition of PDE10 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Inhibition of PDE10 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Antagonist activity at human P2X7 receptor transfected in THP1 cells assessed as inhibition of benzoyl-ATP-induced changes in plasma membrane pore fo...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of PDE9A (unknown origin)More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)