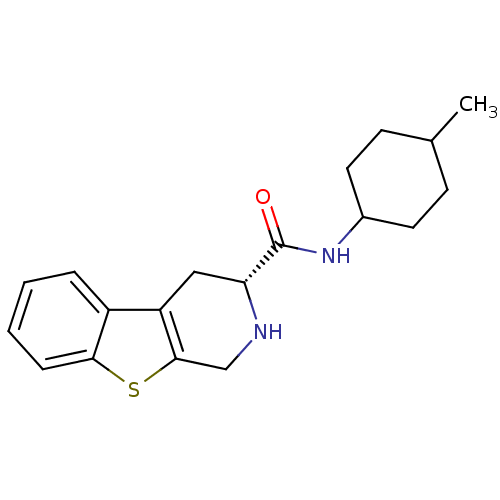
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
5-hydroxytryptamine receptor 1A
Ligand
BDBM50368716
Substrate
n/a
Meas. Tech.
ChEBML_1328
IC50
32±n/a nM
Citation
 Kawakubo, H; Takagi, S; Yamaura, Y; Katoh, S; Ishimoto, Y; Nagatani, T; Mochizuki, D; Kamata, T; Sasaki, Y (R)-1,2,3,4-tetrahydro[1]benzothieno[2,3-c]pyridines: novel optically active compounds with strong 5-HT1A receptor binding ability exhibiting anticonflict activity and lessening of memory impairment. J Med Chem 36:3526-32 (1994) [PubMed] Article
Kawakubo, H; Takagi, S; Yamaura, Y; Katoh, S; Ishimoto, Y; Nagatani, T; Mochizuki, D; Kamata, T; Sasaki, Y (R)-1,2,3,4-tetrahydro[1]benzothieno[2,3-c]pyridines: novel optically active compounds with strong 5-HT1A receptor binding ability exhibiting anticonflict activity and lessening of memory impairment. J Med Chem 36:3526-32 (1994) [PubMed] Article More Info.:
Target
Name:
5-hydroxytryptamine receptor 1A
Synonyms:
5-HT-1A | 5-HT1 | 5-HT1A | 5-Hydroxytryptamine receptor 1A (5-HT1A) | 5-hydroxytryptamine receptor 1A (5HT1A) | 5HT1A_RAT | 5ht1a | G-21 | Htr1a | Serotonin 1 (5-HT1) receptor | Serotonin 1a (5-HT1a) receptor/Adrenergic receptor alpha-1 | Serotonin receptor 1A
Type:
G Protein-Coupled Receptor (GPCR)
Mol. Mass.:
46445.29
Organism:
Rattus norvegicus (rat)
Description:
Binding assays were performed using rat hippocampal membranes.
Residue:
422
Sequence:
MDVFSFGQGNNTTASQEPFGTGGNVTSISDVTFSYQVITSLLLGTLIFCAVLGNACVVAAIALERSLQNVANYLIGSLAVTDLMVSVLVLPMAALYQVLNKWTLGQVTCDLFIALDVLCCTSSILHLCAIALDRYWAITDPIDYVNKRTPRRAAALISLTWLIGFLISIPPMLGWRTPEDRSDPDACTISKDHGYTIYSTFGAFYIPLLLMLVLYGRIFRAARFRIRKTVRKVEKKGAGTSLGTSSAPPPKKSLNGQPGSGDWRRCAENRAVGTPCTNGAVRQGDDEATLEVIEVHRVGNSKEHLPLPSESGSNSYAPACLERKNERNAEAKRKMALARERKTVKTLGIIMGTFILCWLPFFIVALVLPFCESSCHMPALLGAIINWLGYSNSLLNPVIYAYFNKDFQNAFKKIIKCKFCRR
Inhibitor
Name:
BDBM50368716
Synonyms:
CHEMBL1203193
Type:
Small organic molecule
Emp. Form.:
C19H24N2OS
Mol. Mass.:
328.472
SMILES:
CC1CCC(CC1)NC(=O)[C@H]1Cc2c(CN1)sc1ccccc21 |r,wU:10.10,(-11.92,3.89,;-10.82,3.25,;-10.82,1.66,;-9.44,.87,;-8.06,1.66,;-8.06,3.25,;-9.44,4.05,;-6.68,.87,;-5.31,1.67,;-5.31,2.94,;-3.92,.87,;-2.64,1.63,;-1.3,.87,;-1.3,-.64,;-2.64,-1.4,;-3.92,-.64,;,-1.4,;1.3,-.64,;2.62,-1.4,;3.96,-.64,;3.96,.87,;2.62,1.63,;1.3,.87,)|