Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Poly [ADP-ribose] polymerase 1
Ligand
BDBM50434156
Substrate
n/a
Meas. Tech.
ChEMBL_959447 (CHEMBL2384485)
IC50
>85000±n/a nM
Citation
 Bregman, H; Chakka, N; Guzman-Perez, A; Gunaydin, H; Gu, Y; Huang, X; Berry, V; Liu, J; Teffera, Y; Huang, L; Egge, B; Mullady, EL; Schneider, S; Andrews, PS; Mishra, A; Newcomb, J; Serafino, R; Strathdee, CA; Turci, SM; Wilson, C; DiMauro, EF Discovery of novel, induced-pocket binding oxazolidinones as potent, selective, and orally bioavailable tankyrase inhibitors. J Med Chem 56:4320-42 (2013) [PubMed] Article
Bregman, H; Chakka, N; Guzman-Perez, A; Gunaydin, H; Gu, Y; Huang, X; Berry, V; Liu, J; Teffera, Y; Huang, L; Egge, B; Mullady, EL; Schneider, S; Andrews, PS; Mishra, A; Newcomb, J; Serafino, R; Strathdee, CA; Turci, SM; Wilson, C; DiMauro, EF Discovery of novel, induced-pocket binding oxazolidinones as potent, selective, and orally bioavailable tankyrase inhibitors. J Med Chem 56:4320-42 (2013) [PubMed] Article More Info.:
Target
Name:
Poly [ADP-ribose] polymerase 1
Synonyms:
(ARTD1 or PARP1) | 2.4.2.- | 2.4.2.30 | ADP-ribosyltransferase diphtheria toxin-like 1 | ADPRT | ADPRT 1 | ARTD1 | DNA ADP-ribosyltransferase PARP1 | Human diphtheria toxin-like ADP-ribosyltransferase (ARTD1 or PARP1) | NAD(+) ADP-ribosyltransferase 1 | NT-PARP-1 | PARP-1 | PARP1 | PARP1_HUMAN | PPOL | Poly [ADP-ribose] polymerase (PARP) | Poly [ADP-ribose] polymerase 1 (PARP) | Poly [ADP-ribose] polymerase 1 (PARP-1) | Poly [ADP-ribose] polymerase 1 (PARP1) | Poly [ADP-ribose] polymerase 1, 24-kDa form | Poly [ADP-ribose] polymerase 1, 28-kDa form | Poly [ADP-ribose] polymerase 1, 89-kDa form | Poly [ADP-ribose] polymerase 1, processed C-terminus | Poly [ADP-ribose] polymerase 1, processed N-terminus | Poly [ADP-ribose] polymerase-1 | Poly(ADP-ribose) polymerase 1 (PARP1) | Poly(ADP-ribose) polymerase-1 (ARTD1/PARP1) | Poly[ADP-ribose] synthase 1 | Protein poly-ADP-ribosyltransferase PARP1 | Synonyms=ADPRT
Type:
n/a
Mol. Mass.:
113114.22
Organism:
Homo sapiens (Human)
Description:
P09874
Residue:
1014
Sequence:
MAESSDKLYRVEYAKSGRASCKKCSESIPKDSLRMAIMVQSPMFDGKVPHWYHFSCFWKVGHSIRHPDVEVDGFSELRWDDQQKVKKTAEAGGVTGKGQDGIGSKAEKTLGDFAAEYAKSNRSTCKGCMEKIEKGQVRLSKKMVDPEKPQLGMIDRWYHPGCFVKNREELGFRPEYSASQLKGFSLLATEDKEALKKQLPGVKSEGKRKGDEVDGVDEVAKKKSKKEKDKDSKLEKALKAQNDLIWNIKDELKKVCSTNDLKELLIFNKQQVPSGESAILDRVADGMVFGALLPCEECSGQLVFKSDAYYCTGDVTAWTKCMVKTQTPNRKEWVTPKEFREISYLKKLKVKKQDRIFPPETSASVAATPPPSTASAPAAVNSSASADKPLSNMKILTLGKLSRNKDEVKAMIEKLGGKLTGTANKASLCISTKKEVEKMNKKMEEVKEANIRVVSEDFLQDVSASTKSLQELFLAHILSPWGAEVKAEPVEVVAPRGKSGAALSKKSKGQVKEEGINKSEKRMKLTLKGGAAVDPDSGLEHSAHVLEKGGKVFSATLGLVDIVKGTNSYYKLQLLEDDKENRYWIFRSWGRVGTVIGSNKLEQMPSKEDAIEHFMKLYEEKTGNAWHSKNFTKYPKKFYPLEIDYGQDEEAVKKLTVNPGTKSKLPKPVQDLIKMIFDVESMKKAMVEYEIDLQKMPLGKLSKRQIQAAYSILSEVQQAVSQGSSDSQILDLSNRFYTLIPHDFGMKKPPLLNNADSVQAKVEMLDNLLDIEVAYSLLRGGSDDSSKDPIDVNYEKLKTDIKVVDRDSEEAEIIRKYVKNTHATTHNAYDLEVIDIFKIEREGECQRYKPFKQLHNRRLLWHGSRTTNFAGILSQGLRIAPPEAPVTGYMFGKGIYFADMVSKSANYCHTSQGDPIGLILLGEVALGNMYELKHASHISKLPKGKHSVKGLGKTTPDPSANISLDGVDVPLGTGISSGVNDTSLLYNEYIVYDIAQVNLKYLLKLKFNFKTSLW
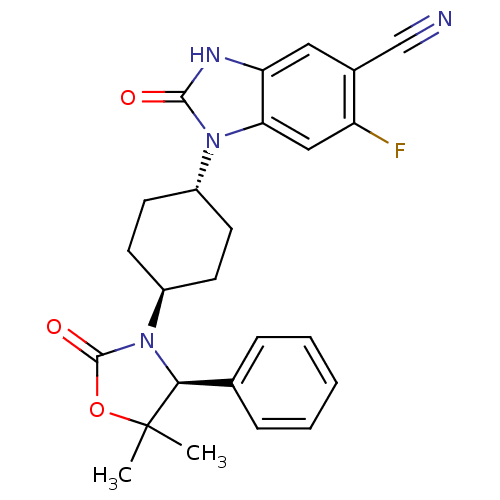
Inhibitor
Name:
BDBM50434156
Synonyms:
CHEMBL2381945 | US9340549, 103
Type:
Small organic molecule
Emp. Form.:
C25H25FN4O3
Mol. Mass.:
448.4894
SMILES:
CC1(C)OC(=O)N([C@H]1c1ccccc1)[C@H]1CC[C@@H](CC1)n1c2cc(F)c(cc2[nH]c1=O)C#N |r,wU:17.22,7.8,wD:14.15,(18.3,-25.15,;19.08,-23.82,;17.54,-23.81,;19.09,-22.28,;20.55,-21.8,;21.03,-20.34,;21.46,-23.05,;20.55,-24.3,;21.38,-25.59,;20.67,-26.95,;21.5,-28.25,;23.04,-28.18,;23.75,-26.81,;22.91,-25.51,;23,-23.05,;23.77,-24.39,;25.31,-24.39,;26.08,-23.05,;25.3,-21.71,;23.76,-21.72,;27.62,-23.05,;28.53,-24.29,;28.21,-25.79,;29.35,-26.82,;29.03,-28.33,;30.82,-26.34,;31.13,-24.84,;29.99,-23.81,;29.99,-22.27,;28.52,-21.8,;28.04,-20.33,;31.97,-27.36,;33.12,-28.39,)|