Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Lysosomal acid glucosylceramidase
Ligand
BDBM18355
Substrate
n/a
Meas. Tech.
ChEMBL_1435475 (CHEMBL3384690)
IC50
400000±n/a nM
Citation
 Ghisaidoobe, AT; van den Berg, RJ; Butt, SS; Strijland, A; Donker-Koopman, WE; Scheij, S; van den Nieuwendijk, AM; Koomen, GJ; van Loevezijn, A; Leemhuis, M; Wennekes, T; van der Stelt, M; van der Marel, GA; van Boeckel, CA; Aerts, JM; Overkleeft, HS Identification and development of biphenyl substituted iminosugars as improved dual glucosylceramide synthase/neutral glucosylceramidase inhibitors. J Med Chem 57:9096-104 (2014) [PubMed] Article
Ghisaidoobe, AT; van den Berg, RJ; Butt, SS; Strijland, A; Donker-Koopman, WE; Scheij, S; van den Nieuwendijk, AM; Koomen, GJ; van Loevezijn, A; Leemhuis, M; Wennekes, T; van der Stelt, M; van der Marel, GA; van Boeckel, CA; Aerts, JM; Overkleeft, HS Identification and development of biphenyl substituted iminosugars as improved dual glucosylceramide synthase/neutral glucosylceramidase inhibitors. J Med Chem 57:9096-104 (2014) [PubMed] Article More Info.:
Target
Name:
Lysosomal acid glucosylceramidase
Synonyms:
Acid beta-glucosidase | Alglucerase | Beta-glucocerebrosidase | Beta-glucocerebrosidase (GC) | D-glucosyl-N-acylsphingosine glucohydrolase | GBA | GBA1 | GBA1_HUMAN | GC | GCase | GLUC | Glucocerebrosidase (GBA) | Glucosylceramidase (GBA) | Glucosylceramidase (GCase) | Glucosylceramidase precursor (Beta-glucocerebrosidase) (Acid beta-glucosidase) (D-glucosyl-N-acylsphingosine glucohydrolase) (Alglucerase) (Imiglucerase) | Imiglucerase | beta-glucocerebrosidase (GCase)
Type:
Enzyme
Mol. Mass.:
59724.64
Organism:
Homo sapiens (Human)
Description:
The beta-Glu activity was measured with commercially available beta-glucocerebrosidase (Ceredase) as the enzyme source.
Residue:
536
Sequence:
MEFSSPSREECPKPLSRVSIMAGSLTGLLLLQAVSWASGARPCIPKSFGYSSVVCVCNATYCDSFDPPTFPALGTFSRYESTRSGRRMELSMGPIQANHTGTGLLLTLQPEQKFQKVKGFGGAMTDAAALNILALSPPAQNLLLKSYFSEEGIGYNIIRVPMASCDFSIRTYTYADTPDDFQLHNFSLPEEDTKLKIPLIHRALQLAQRPVSLLASPWTSPTWLKTNGAVNGKGSLKGQPGDIYHQTWARYFVKFLDAYAEHKLQFWAVTAENEPSAGLLSGYPFQCLGFTPEHQRDFIARDLGPTLANSTHHNVRLLMLDDQRLLLPHWAKVVLTDPEAAKYVHGIAVHWYLDFLAPAKATLGETHRLFPNTMLFASEACVGSKFWEQSVRLGSWDRGMQYSHSIITNLLYHVVGWTDWNLALNPEGGPNWVRNFVDSPIIVDITKDTFYKQPMFYHLGHFSKFIPEGSQRVGLVASQKNDLDAVALMHPDGSAVVVVLNRSSKDVPLTIKDPAVGFLETISPGYSIHTYLWRRQ
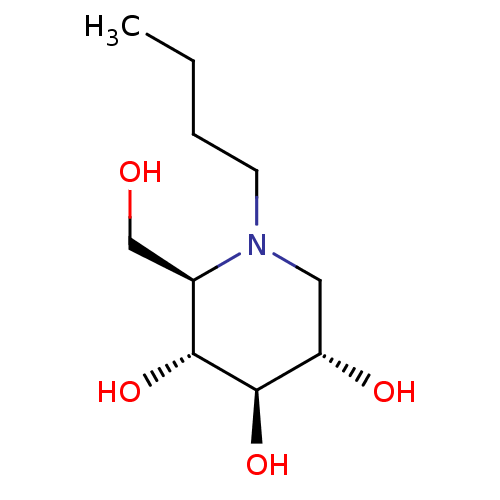
Inhibitor
Name:
BDBM18355
Synonyms:
(2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-3,4,5-triol | CHEMBL1029 | MIGLUSTAT | N-Butyl-DNJ | US20230339856, Compound NB-DNJ | US9181184, 5
Type:
Small organic molecule
Emp. Form.:
C10H21NO4
Mol. Mass.:
219.278
SMILES:
CCCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO