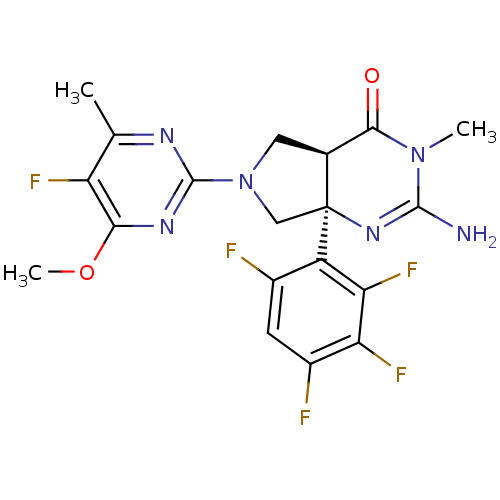
BDBM102958 US8541427, 29
SMILES COc1nc(nc(C)c1F)N1C[C@H]2C(=O)N(C)C(N)=N[C@]2(C1)c1c(F)cc(F)c(F)c1F
InChI Key InChIKey=RQQNLWISWSTQLK-RLBGWGEZSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 102958
Found 3 hits for monomerid = 102958
Affinity DataKi: 30nM ΔG°: -10.4kcal/mole IC50: 93nMT: 2°CAssay Description:A homogeneous time-resolved FRET assay was used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. The assay monit...More data for this Ligand-Target Pair
Affinity DataIC50: 93nMAssay Description:Inhibition of BACE1 in human HEK293 cells transfected with human APP Swe/Lon mutations assessed as amyloid beta 40 level after 4 hrs by electrochemil...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP3A4 in human liver microsomes using testosterone and midazolam as substrate preincubated for 30 mins followed substrate addition by ...More data for this Ligand-Target Pair