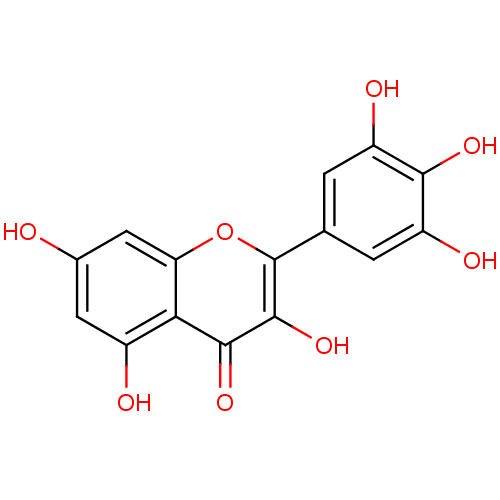
BDBM15236 3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chromen-4-one::3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)chromen-4-one::CHEMBL164::Cannabiscetin::Myricetin::Myricetin (20)::Myricetin (Myr)::cid_5281672
SMILES Oc1cc(O)c2c(c1)oc(-c1cc(O)c(O)c(O)c1)c(O)c2=O
InChI Key InChIKey=IKMDFBPHZNJCSN-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 99 hits for monomerid = 15236
Found 99 hits for monomerid = 15236
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Mrc
Mrc
Affinity DataIC50: 1.80E+3nM Kd: 170nMpH: 7.2 T: 2°CAssay Description:Binding was detected as a change in the intrinsic tryptophan fluorescence of the PI3K upon the addition of inhibitor. The inhibitor was incubated wit...More data for this Ligand-Target Pair
Affinity DataIC50: 3.02E+4nMpH: 6.0 T: 2°CAssay Description:The assay was carried out at room temperature for 10 min with salivary alpha-amylase, starch, and test compounds. The reducing sugar was determined b...More data for this Ligand-Target Pair
Affinity DataIC50: 780nMpH: 7.0 T: 2°CAssay Description:The 96-well flat-bottomed plates were coated with recombinant GST-BAD. After the plates were blocked, the reaction buffer containing test compound an...More data for this Ligand-Target Pair
TargetAlkaline phosphatase, tissue-nonspecific isozyme(Homo sapiens (Human))
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 3.32E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetIntestinal-type alkaline phosphatase 1(Rattus norvegicus (Rat))
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 1.25E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetDNA dC->dU-editing enzyme APOBEC-3G(Homo sapiens (Human))
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 2.39E+3nMT: 2°CAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetDNA dC->dU-editing enzyme APOBEC-3A(Homo sapiens (Human))
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 1.75E+4nMT: 2°CAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
Eberhard Karls University of Tuebingen
Eberhard Karls University of Tuebingen
Affinity DataIC50: 1.64E+3nMT: 2°CAssay Description:The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Homo sapiens (Human))
Eberhard Karls University of Tuebingen
Eberhard Karls University of Tuebingen
Affinity DataIC50: 2.24E+3nMT: 2°CAssay Description:The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n...More data for this Ligand-Target Pair
Affinity DataIC50: 1.01E+5nMpH: 7.45 T: 2°CAssay Description:Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione.More data for this Ligand-Target Pair
TargetHuntingtin(Homo sapiens (Human))
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
TargetPC4 and SFRS1-interacting protein(Homo sapiens (Human))
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataIC50: 1.14E+3nMAssay Description:Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Affiliation: Ohio State University Assay Provider: Mam...More data for this Ligand-Target Pair
TargetPC4 and SFRS1-interacting protein(Homo sapiens (Human))
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataIC50: 758nMAssay Description:Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Affiliation: Ohio State University Assay Provider: Mam...More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:H-DXR was pre-incubated during 2 min in the presence of the inhibitors at different concentrations and DXP (480 ÁM). NADPH (160 ÁM final concentratio...More data for this Ligand-Target Pair
Affinity DataIC50: 3.98E+3nMAssay Description:The kinase assay was performed using the EMD Millipore KinaseProfiler service assay protocol. Aurora B kinase was supplied by EMD Millipore Corp. The...More data for this Ligand-Target Pair
Affinity DataIC50: 1.51E+4nMpH: 7.4 T: 2°CAssay Description:Fluorescence intensity was measured at 420 nm excitation and 485 nm emission using a microplate reader (MPR-A4╬╣II; TOSOH, Tokyo, Japan, or Fluoroska...More data for this Ligand-Target Pair
TargetInterstitial collagenase [100-268](Homo sapiens (Human))
East China University of Science and Technology
East China University of Science and Technology
Affinity DataIC50: 2.92E+3nMpH: 7.5Assay Description:The activity of cd-MMP-1 was measured using a fluorescence-based assay. It was performed in white 96-well half area microplate (Greiner) in a final v...More data for this Ligand-Target Pair
Affinity DataIC50: 776nMAssay Description:Inhibition of PIM1 kinaseChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+3nMAssay Description:IC50 was measured as concentration required to inhibit 50% of HIV-integrase integrationMore data for this Ligand-Target Pair
Affinity DataIC50: 1.02E+4nMAssay Description:Inhibitory concentration of the compounds against Bovine trypsin enzyme.More data for this Ligand-Target Pair
TargetMultidrug resistance-associated protein 1(Homo sapiens (Human))
The Hong Kong Polytechnic University
Curated by ChEMBL
The Hong Kong Polytechnic University
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of MRP1More data for this Ligand-Target Pair
TargetATP-binding cassette sub-family C member 2(Homo sapiens (Human))
The Hong Kong Polytechnic University
Curated by ChEMBL
The Hong Kong Polytechnic University
Curated by ChEMBL
Affinity DataIC50: 2.20E+4nMAssay Description:Inhibition of MRP2More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Mrc
Mrc
Affinity DataIC50: 4.72E+3nMAssay Description:Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrateMore data for this Ligand-Target Pair
TargetMicrotubule-associated protein tau(Homo sapiens (Human))
Tokyo Institute Of Technology
Curated by ChEMBL
Tokyo Institute Of Technology
Curated by ChEMBL
Affinity DataIC50: <3.00E+3nMAssay Description:Inhibition of His-tagged human brain tau 3R MBD aggregation after 16 hrs by thioflavin T fluorescence methodMore data for this Ligand-Target Pair
Affinity DataIC50: 560nMAssay Description:Inhibition of human recombinant His-tagged Glyoxalase 1 expressed in Sf21-Baculovirus systemMore data for this Ligand-Target Pair
Affinity DataIC50: 2.30E+4nMAssay Description:Displacement of ANS from DAPK1 catalytic domain (1 to 285) (unknown origin) after 30 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.51E+4nMAssay Description:Inhibition of wild type Amyloid beta (1 to 42) (unknown origin) aggregation by Thioflavin-T fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+5nMAssay Description:Inhibition of human Neu2 assessed as MuNANA substrate hydrolysis in presence of 0.1% Triton X-100 by discontinuous fluorimetric assayMore data for this Ligand-Target Pair
TargetADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1(Homo sapiens (Human))
University Of Strasburg
Curated by ChEMBL
University Of Strasburg
Curated by ChEMBL
Affinity DataIC50: 2.48E+4nMAssay Description:Inhibition of human CD38 using 20 uM 1, N6-etheno NAD+ as substrate by continuous fluorimetric methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of JMJD2EMore data for this Ligand-Target Pair
Affinity DataIC50: 560nMAssay Description:Inhibition of recombinant human His-tagged glyoxalase 1 expressed in Escherichia coli BL21 assessed as formation of S-D-lactoylglutathione after 5 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 7.60E+3nMAssay Description:IC50 was measured as concentration required to inhibit 50% of HIV-integrase cleavageMore data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Tested for inhibition of HIV-1 integrase, under 1 uM for the strand transferMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of HIV-1 integrase, under 1 uM for the 3''-preprocessingMore data for this Ligand-Target Pair
TargetMO15-related protein kinase Pfmrk(Plasmodium falciparum)
Walter Reed Army Institute Of Research
Curated by ChEMBL
Walter Reed Army Institute Of Research
Curated by ChEMBL
Affinity DataIC50: 1.57E+5nMAssay Description:Inhibition of Plasmodium falciparum cyclin-dependent kinaseMore data for this Ligand-Target Pair
Target3-oxoacyl-acyl-carrier protein reductase(Plasmodium falciparum)
University Of Zurich
Curated by ChEMBL
University Of Zurich
Curated by ChEMBL
Affinity DataIC50: 1.40E+4nMAssay Description:Inhibition of FabGMore data for this Ligand-Target Pair
TargetEnoyl-acyl-carrier protein reductase(Plasmodium falciparum)
University Of Zurich
Curated by ChEMBL
University Of Zurich
Curated by ChEMBL
Target3-hydroxyacyl-[acyl-carrier-protein] dehydratase(Plasmodium falciparum)
University Of Zurich
Curated by ChEMBL
University Of Zurich
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of FabZMore data for this Ligand-Target Pair
Affinity DataIC50: 6.40E+3nMAssay Description:Inhibition of SYKMore data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+5nMAssay Description:Inhibition of Escherichia coli primaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substrate incubated f...More data for this Ligand-Target Pair
TargetMicrotubule-associated protein tau(Homo sapiens (Human))
Tokyo Institute Of Technology
Curated by ChEMBL
Tokyo Institute Of Technology
Curated by ChEMBL
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of human recombinant brain tau protein (412 amino acid residues) filament assembly expressed in Escherichia coli BL21(DE3) by electron mic...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
Dongguk University-Seoul
Curated by ChEMBL
Dongguk University-Seoul
Curated by ChEMBL
Affinity DataIC50: 1.69E+3nMAssay Description:Inhibition of recombinant FLT3 (unknown origin) by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.38E+3nMAssay Description:Inhibition of xanthine oxidase assessed as decrease in uric acid production by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 1.23E+4nMAssay Description:Inhibition of mouse recombinant AKR1C21More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
Monash University
Curated by ChEMBL
Monash University
Curated by ChEMBL
Affinity DataIC50: 2.90E+4nMAssay Description:Inhibition of human recombinant aldose reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human aromatase by fluorometric assayMore data for this Ligand-Target Pair
TargetProtein E6(Human papillomavirus type 16)
Loma Linda University School Of Medicine
Curated by ChEMBL
Loma Linda University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: 850nMAssay Description:Inhibition of GST-tagged Human papillomavirus 16 protein E6 interaction with His-tagged human caspase 8 expressed in Escherichia coli after 1 hr incu...More data for this Ligand-Target Pair
Affinity DataIC50: 2.12E+3nMAssay Description:Desensitization of GPR35 receptor in human HT-29 cells assessed as inhibition of zaprinast-induced dynamic mass redistribution after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 7.07E+5nMAssay Description:In vitro antibacterial activity was determined as inhibitory concentration causing 50% DNA-gyrase supercoiling inhibition (SCI)More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)