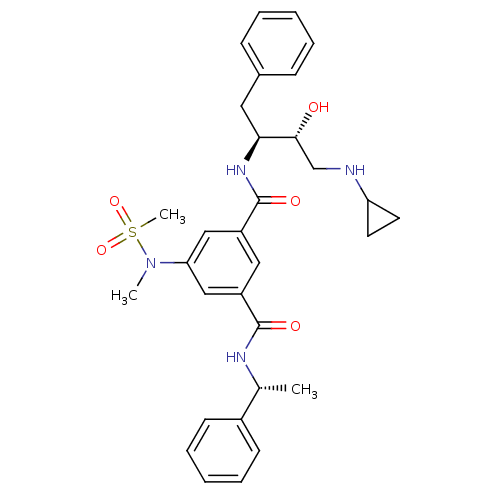
BDBM16034 1-N-[(2S,3R)-4-(cyclopropylamino)-3-hydroxy-1-phenylbutan-2-yl]-5-(N-methylmethanesulfonamido)-3-N-[(1R)-1-phenylethyl]benzene-1,3-dicarboxamide::5-Substituted isophthalamide, 1::CHEMBL378225::Isophthalamide Derivative 24::L-000384950::N-[(1S,2R)-1-benzyl-3-(cyclopropylamino)-2-hydroxypropyl]-5-[methyl(methylsulfonyl)amino]-N-[(1R)-1-phenylethyl]benzene-1,3-dicarboxamide::hydroxyethylamine (HEA) derived inhibitor 1
SMILES C[C@@H](NC(=O)c1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC1CC1)N(C)S(C)(=O)=O)c1ccccc1
InChI Key InChIKey=VPNIQGRFZCTBEZ-SPTGULJVSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 16034
Found 3 hits for monomerid = 16034
Affinity DataKi: 233nM ΔG°: -9.41kcal/molepH: 4.5 T: 2°CAssay Description:Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record...More data for this Ligand-Target Pair
Affinity DataKi: 240nM ΔG°: -9.39kcal/molepH: 4.5 T: 2°CAssay Description:Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record...More data for this Ligand-Target Pair
Affinity DataKi: 448nM ΔG°: -9.00kcal/molepH: 4.5 T: 2°CAssay Description:Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)