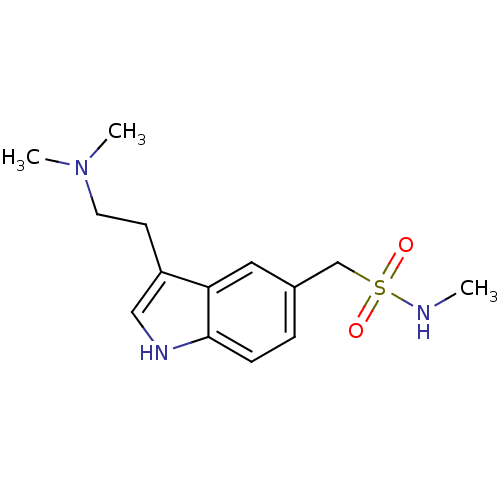
BDBM50005835 (3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methylmethanesulfonamide::1-[3-(2-dimethylaminoethyl)-1H-indol-5-yl]-N-methyl-methanesulfonamide::1-{3-[2-(dimethylamino)ethyl]-1H-indol-5-yl}-N-methylmethanesulfonamide::3-(2-(dimethylamino)ethyl)-N-methyl-1H-indole-5-methanesulfonamide::3-[2-(dimethylamino)ethyl]-N-methylindole-5-methanesulfonamide::CHEMBL128::SUMATRIPTAN::Sumatran::Sumax
SMILES CNS(=O)(=O)Cc1ccc2[nH]cc(CCN(C)C)c2c1
InChI Key InChIKey=KQKPFRSPSRPDEB-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50005835
Found 3 hits for monomerid = 50005835
Target5-hydroxytryptamine receptor 1B(Homo sapiens (Human))
Virginia Commonwealth University
Curated by ChEMBL
Virginia Commonwealth University
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligandMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1A(Homo sapiens (Human))
Virginia Commonwealth University
Curated by ChEMBL
Virginia Commonwealth University
Curated by ChEMBL
Affinity DataKi: 330nMAssay Description:In vitro affinity at human cloned 5-hydroxytryptamine 1A receptor by [3H]-8-OH-DPAT displacement.More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1D(Homo sapiens (Human))
Virginia Commonwealth University
Curated by ChEMBL
Virginia Commonwealth University
Curated by ChEMBL
Affinity DataEC50: 220nMAssay Description:Compound was tested for 5-hydroxytryptamine 1D like receptor-mediated vascular effect in rabbit saphenous vein (RSV)More data for this Ligand-Target Pair